Background
The proton motive force (PMF) lies at the core of energy metabolism, fuelling a wide range of cellular processes. It is a universal phenomenon, comparable to the genetic code, and has played a significant role in shaping evolution. The PMF represents an electrochemical gradient across a membrane, typically generated by the coordinated activity of multiple membrane protein complexes. It serves to separate energy transformation from molecular identities and stoichiometric constraints, enabling the seamless integration of diverse cellular processes. This unique feature has been instrumental in the success of the PMF.
While the PMF exhibits remarkable flexibility, it must also maintain strict reliability to sustain cellular structures and fuel biochemical reactions. To ensure a constant energy supply under variable conditions, environmental and physiological stimuli need to be integrated into PMF regulation. Despite extensive research on the PMF, our understanding of its regulatory strategies remains incomplete.
Recent advancements in functional imaging and biosensing techniques have revealed new and fundamental insights into the mitochondrial PMF. These discoveries have begun to challenge our existing understanding of bioenergetic dynamics. However, similar insights are lacking for oxygenic photosynthesis. Studying the PMF within the context of photosynthesis is particularly suitable for understanding the underlying principles of its dynamics, as photosynthesis is highly susceptible to rapid external changes caused by fluctuating light in natural environments.
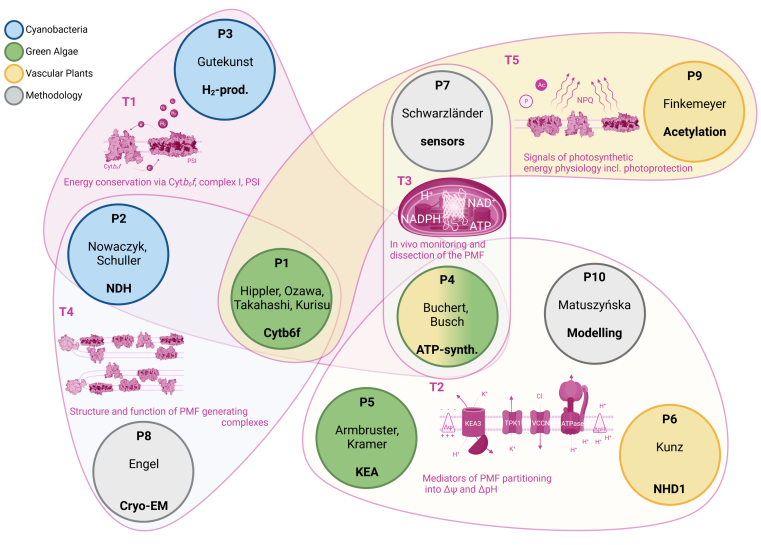
The goal of this Research Group (GoPMF) is to develop conceptual frameworks for understanding the regulation of PMF generation and modulation in order to optimize photosynthetic output in dynamic natural environments. Building upon recent discoveries and methodological advancements achieved by members of the research group, GoPMF investigates photosynthetic bioenergetics within the context of subcellular organization and physiology. GoPMF utilizes cyanobacteria and chloroplasts as in vivo models to dissect the regulatory mechanisms involved in rapid PMF adjustments at the posttranslational and physiological level. GoPMF complements these findings by mechanistic and structural analyses of the molecular machinery responsible for generating and modulating the PMF.
By combining cutting-edge imaging techniques with the development of in situ biosensing methods to monitor the bioenergetic characteristics of the PMF live in individual cells, organelles, and thylakoids, GoPMF aims to explore PMF management within a novel cell biological context. GoPMF gains molecular insights into the mechanisms driving PMF dynamics through fast time-resolved spectroscopy, mass spectrometry, and structural biology, including cryo-electron microscopy/tomography. Extensive genetic engineering approaches leverage the mechanistic conservation and diversity in PMF regulation found in cyanobacteria, algae, and plants. These functional studies are complemented by mathematical modelling of the PMF.
The ultimate goal of GoPMF is to establish a comprehensive understanding of the PMF as a dynamic, responsive, and integrated hub that shapes photosynthesis and enables its adaptation to rapid external changes.