N-Heterocyclic Carbenes (NHC) on Surfaces

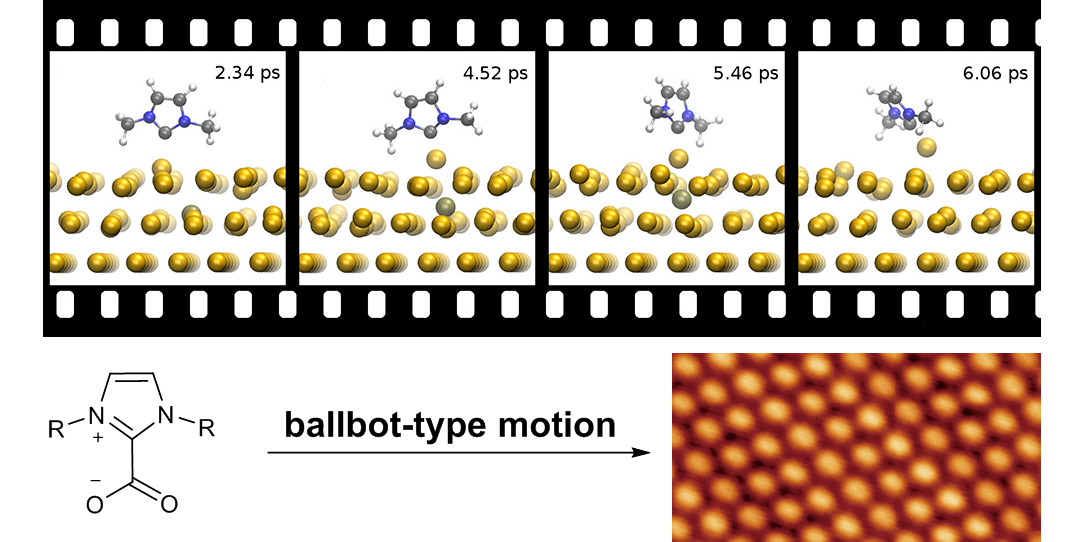
N-Heterocyclic Carbenes (NHCs) are powerful ligands in catalysis due to their strong electron-donating properties and their ability to form very stable bonds to transition metals.[1] Thus, they are used as ligands to stabilize and modify nanoparticles or flat metals surfaces, since they outperform well established phosphine or thiol ligands regarding structural flexibility, electron-donating properties and stability. As an example, our group discovered the ballbot-type motion of NHCs on metal surfaces[2] or the catalyst activation for a heterogeneous Buchwald-Hartwig reaction.[3] Future work in this area will include studies about the fundamental behavior of NHCs on surfaces (rotation, electronic activation, 2D networks) and fine tuning of heterogeneous catalysts towards higher activity, selectivity and stability.
[1] M. N. Hopkinson, C. Richter, M. Schedler, F. Glorius, Nature 2014, 510, 485. [2] G. Wang, A. Rühling, S. Amirjalayer, M. Know, J. B. Ernst, C. Richter, H. J. Gao, A. Timmer, H. Y. Gao, N. L. Doltsinis, F. Glorius, H. Fuchs, Nat. Chem. 2017, 9, 152. [3] J. Ernst, C. Schwermann, G.-I. Yokota, M. Tada, S. Muratsugu, N. L. Doltsinis, F. Glorius, J. Am. Chem. Soc. 2017, 139, 9144.
Please click on the graphical abstracts to come to the original publication
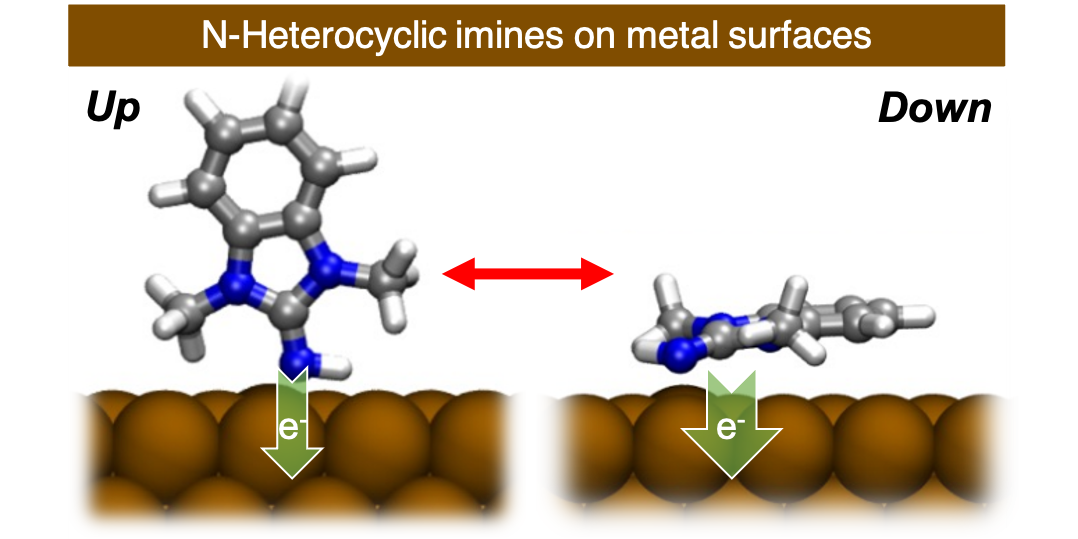
J. Ren,*§ M. Das,§ H. Osthues, M. Nyenhuis, B. Schulze Lammers, E. Kolodzeiski, H. Mönig, S. Amirjalayer,* H. Fuchs,* N. L. Doltsinis,* F. Glorius,*
The electron-rich and nucleophilic N-heterocyclic imines on metal surfaces: binding modes and interfacial charge transfer,
J. Am. Chem. Soc. 2024, 146, 7288-7294.
§ These authors contributed equally.
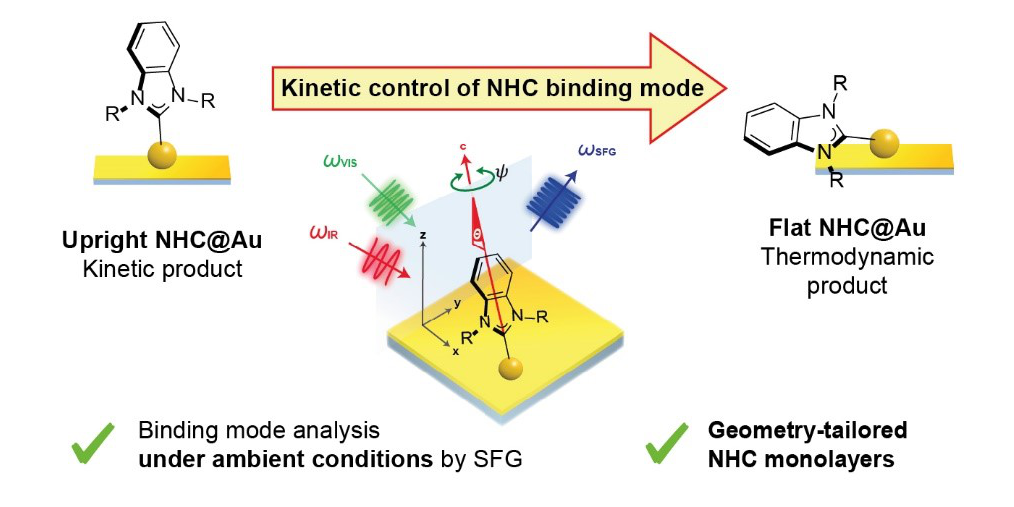
C. Gutheil, G. Ross, S. Amirjalayer, B. Mo, A. H. Schäfer, N. L. Doltsinis, B. Braunschweig,* F. Glorius,*
Tailored Monolayers of N-Heterocyclic Carbenes by Kinetic Control,
ACS Nano 2024, 18, 3043-3052.
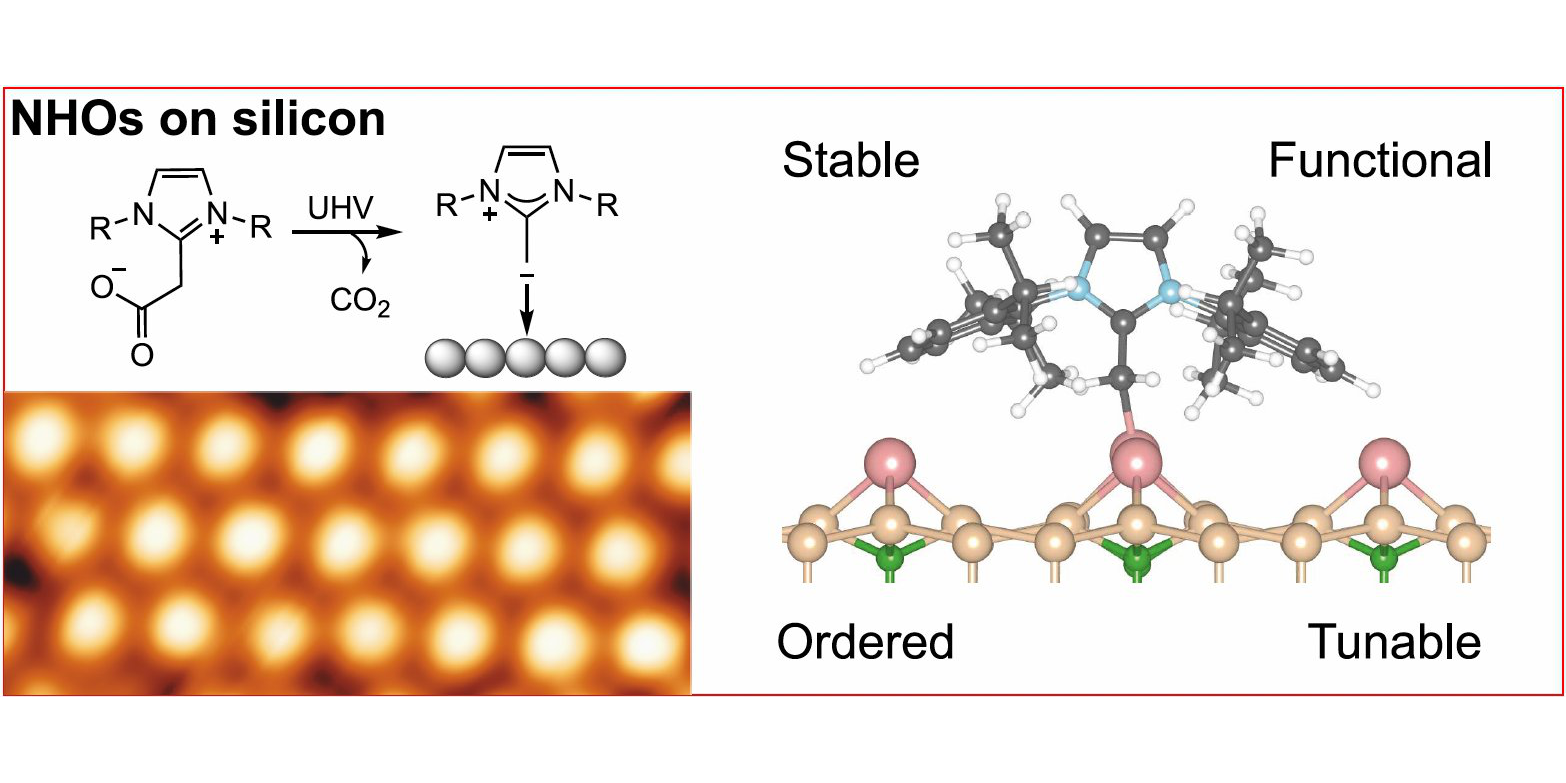
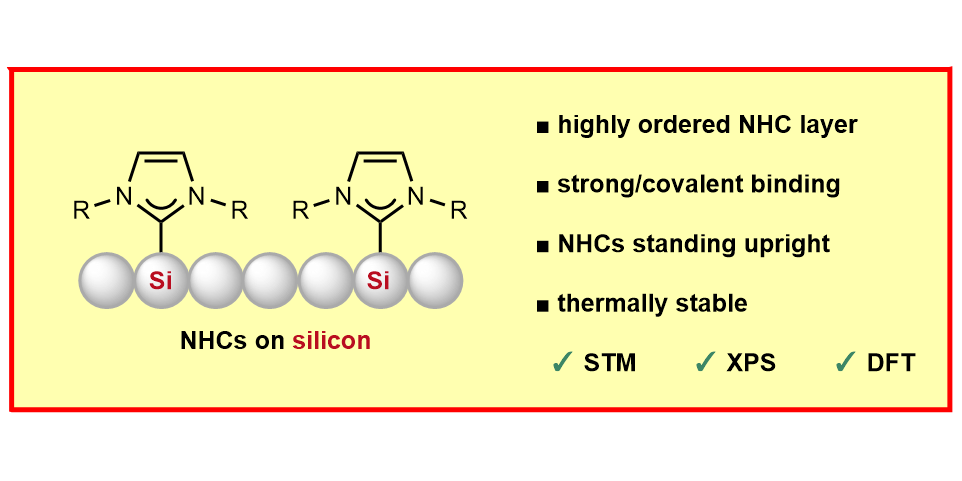
M. Das,§ C. Hogan,§ R. Zielinski, M. Kubicki, M. Koy, C. Kosbab, S. Brozzesi, A. Das, M. T. Nehring, V. Balfanz, J. Brühne, M. Dähne, M. Franz,* N. Esser,* F. Glorius,*
N-Heterocyclic Olefins on a Silicon Surface,
Angew. Chem. Int. Ed. 2023, 62, e202314663; Angew. Chem. 2023, 135, e202314663. DOI
§ These authors contributed equally.
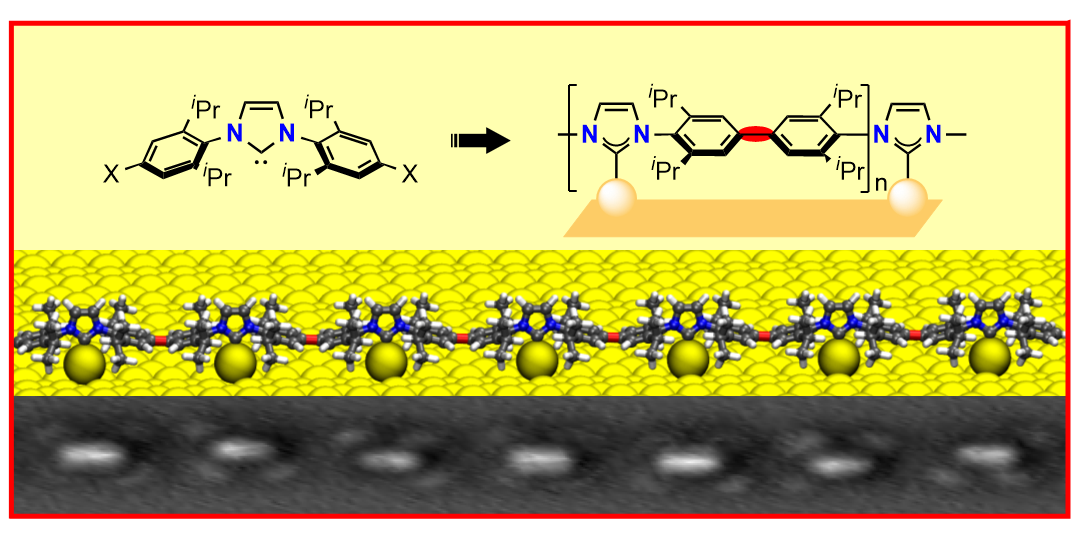
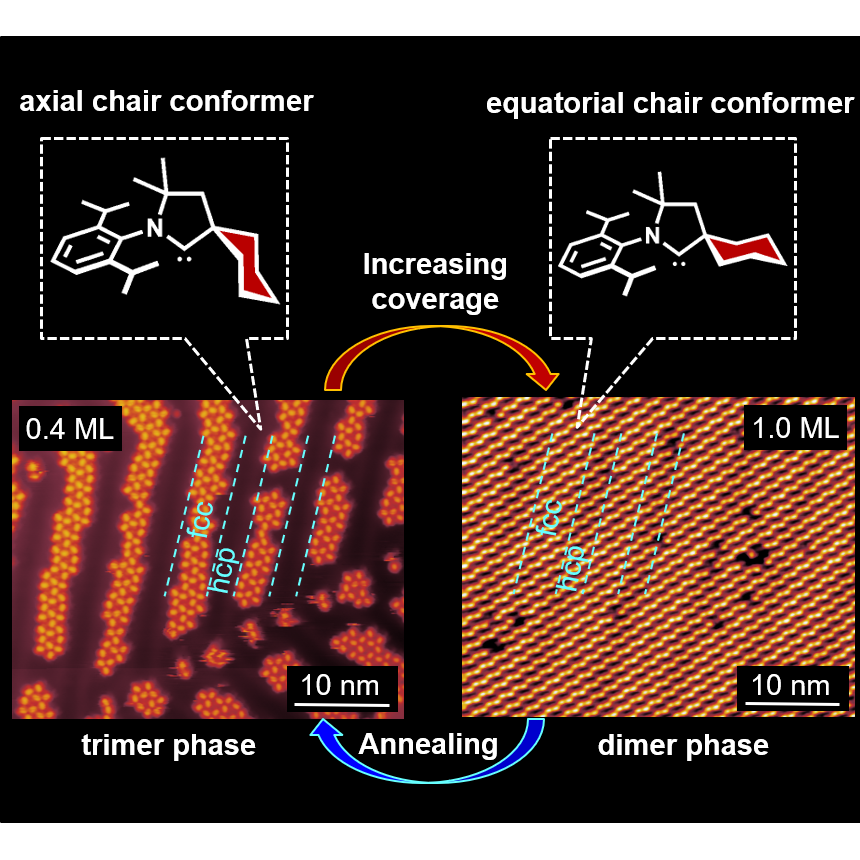
J. Ren, M. Koy, H. Osthues, B. Schulze Lammers, C. Gutheil, M. Nyenhuis, Q. Zheng, Y. Xiao, L. Huang, A. Nalop, Q. Dai, H.-J. Gao,* H. Mönig,* N. L. Doltsinis,* H. Fuchs,* F. Glorius,*
On-surface synthesis of ballbot-type N-heterocyclic carbene polymers,
Nat. Chem. 2023, 15, 1737-1744.
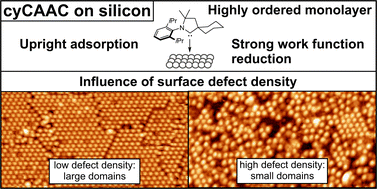
R. Zielinski, M. Das, C. Kosbab, M. T. Nehring, M. Dähne, N. Esser,* M. Franz*, F. Glorius,*
Influence of the defect density on the ordering of an NHC monolayer on a silicon surface,
J. Mater. Chem. C 2023, 11, 7377-7382.
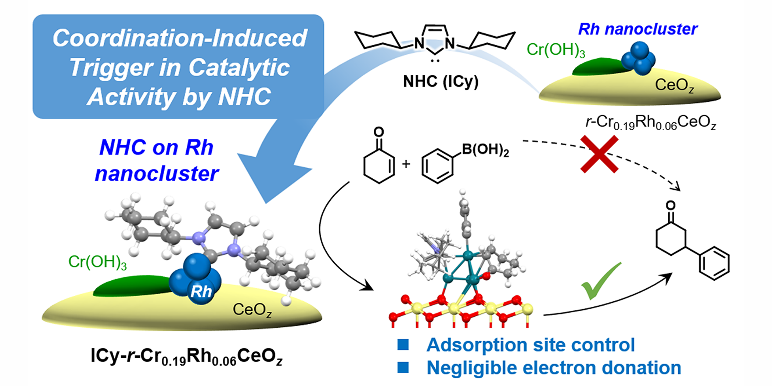
S. Ikemoto, S. Muratsugu,* T. Koitaya, Y. Tsuj, M. Das, K. Yoshizawa, F. Glorius, M. Tada,*
Coordination-Induced Trigger for Activity: N‑Heterocyclic Carbene-Decorated Ceria Catalysts Incorporating Cr and Rh with Activity Induction by Surface Adsorption Site Control,
J. Am. Chem. Soc. 2023, 145, 1497-1504.
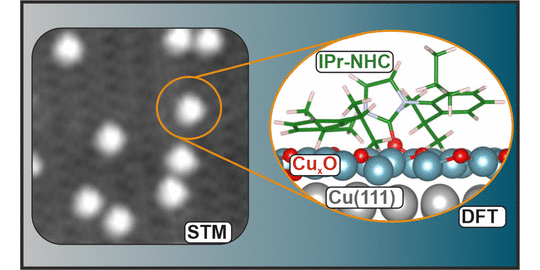
J. J. Navarro, M. Das, S. Tosoni*, F. Landwehr, J. P. Bruce, M. Heyde*, G. Pacchioni, F. Glorius*, B. Roldan Cuenya,
Covalent Adsorption of N-Heterocyclic Carbenes on a Copper Oxide Surface,
J. Am. Chem. Soc. 2022, 144, 16267-16271.
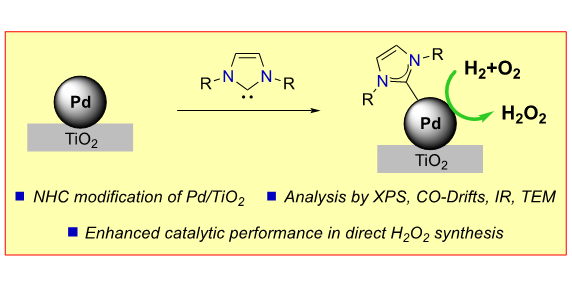
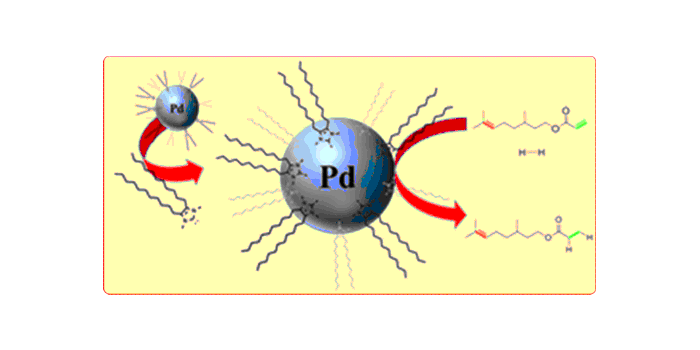
R. J. Lewis,§ M. Koy,§ M. Macino, M. Das, J. H. Carter, D. J. Morgan, T. E. Davies, J. B. Ernst, S. J. Freakley, F. Glorius*, G. J. Hutchings*,
N-Heterocyclic Carbene Modified Palladium Catalysts for the Direct Synthesis of Hydrogen Peroxide,
J. Am. Chem. Soc. 2022, 144, 15431-15436.
§ These authors contributed equally
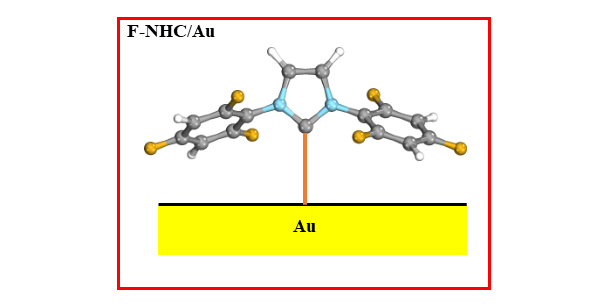
Z. Wang, M. Das, C. Gutheil, H. Osthues, F. Strieth-Kalthoff, A. Timmer, N. L. Doltsinis,* W. Wang,* L Chi,* F. Glorius,*
Surface Modification with a Fluorinated N-Heterocyclic Carbene on Au: Effect on Contact Resistance in Organic Field Effect Transistors,
J. Mater. Chem. C 2022, 10, 8589-8595.
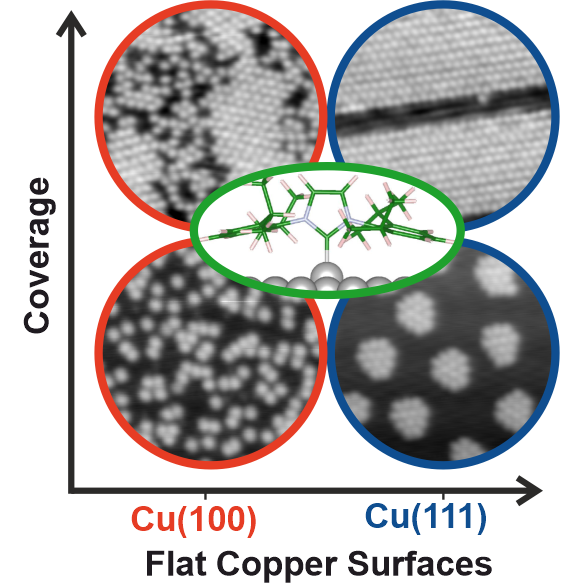
J. J. Navarro, M. Das, S. Tosoni,* F. Landwehr, M. Koy, M. Heyde,* G. Pacchioni, F. Glorius,* B. Roldan Cuenya,
Growth of N-Heterocyclic Carbene Assemblies on Cu(100) and Cu(111): from Single Molecules to Magic-Number Islands,
Angew. Chem. Int. Ed. 2022, 61 (30), e202202127; Angew. Chem. 2022, 134 (30), e202202127.
J. Ren,§ M. Freitag,§ Y. Gao,§ P. Bellotti, M. Das, B. Schulze Lammers, H. Mönig, Y. Zhang, C. G. Daniliuc, S. Du*, H. Fuchs*, F. Glorius,*
Reversible Self-Assembly of an N-Heterocyclic Carbene on Metal Surfaces,
Angew. Chem. Int. Ed. 2022, 61, e202115104.
§ These authors contributed equally
P. Bellotti, M. Koy, M. N. Hopkinson,* F. Glorius,*
Recent advances in the chemistry and applications of N-heterocyclic carbenes,
Nat. Rev. Chem. 2021, 5, 711-725.
M. Franz, S. Chandola, M. Koy, R. Zielinski, H. Aldahhak, M. Das, M. Freitag, U. Gerstmann, D. Liebig, A. K.Hoffmann, M. Rosin, W. G. Schmidt, C. Hogan, F. Glorius,* N. Esser,* M. Dähne,*
Controlled growth of ordered monolayers of N-heterocyclic carbenes on silicon,
Nature Chem. 2021, 13, 828-835.
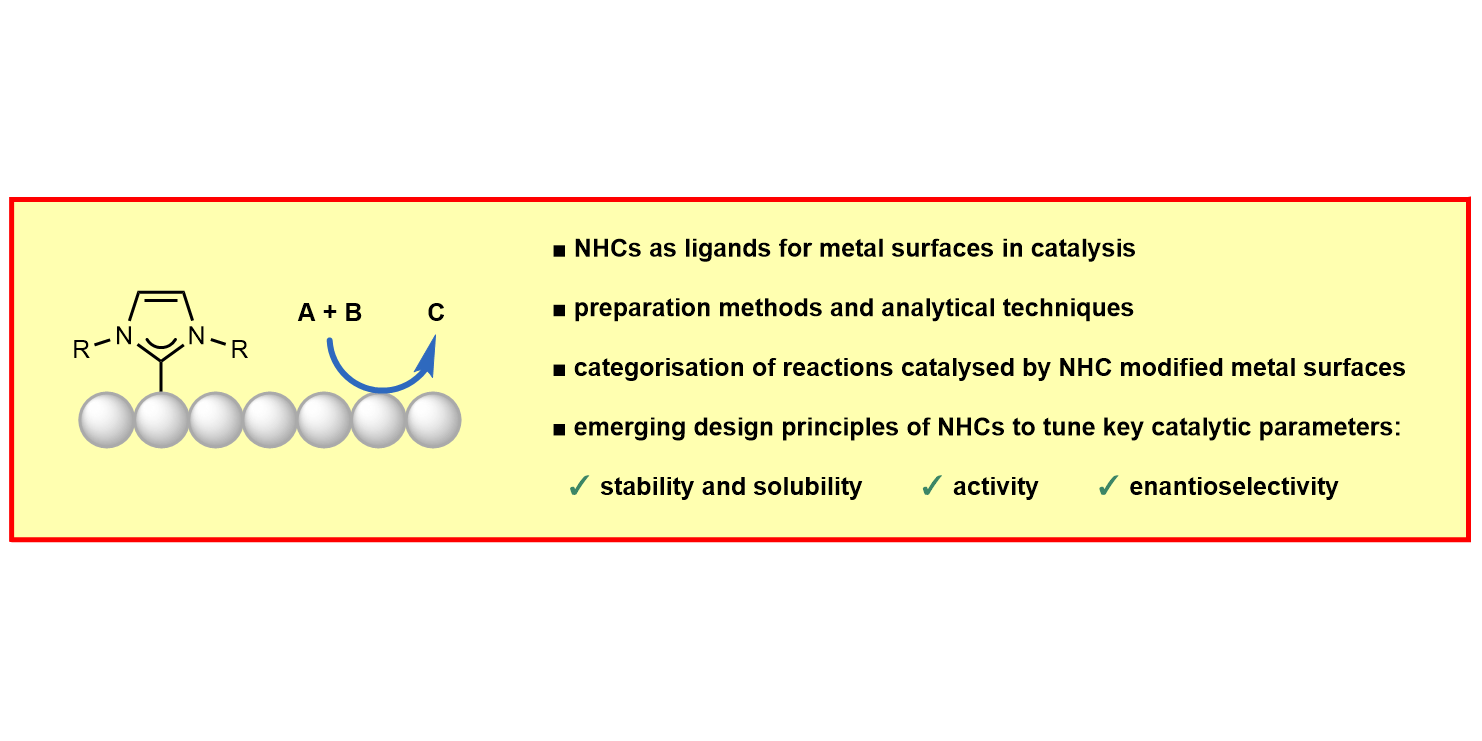
M. Koy, P. Bellotti, M. Das, F. Glorius,
N-Heterocyclic carbenes as tunable ligands for catalytic metal surfaces,
Nature Catal. 2021, 4, 352-363.
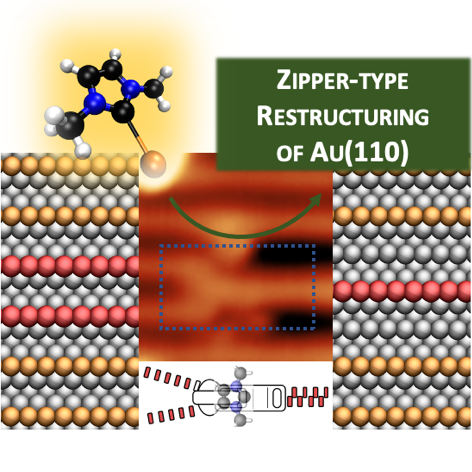
S. Amirjalayer,* A. Bakker, M. Freitag, F. Glorius, H. Fuchs,
Cooperation of N‐Heterocyclic Carbenes on a Gold Surface,
Angew. Chem. Int. Ed. 2020, 59, 21230-21235; Angew. Chem. 2020, 132, 21416-21422.
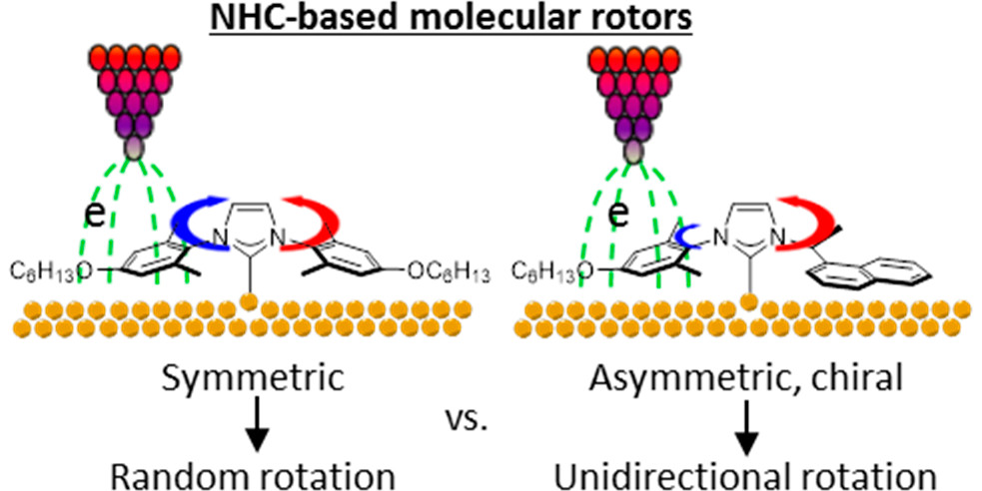
J. Ren, M. Freitag, C. Schwermann, A. Bakker, S. Amirjalayer, A. Rühling, H.-Y. Gao, N. L. Doltsinis*, F. Glorius*, H. Fuchs*,
A Unidirectional Surface-Anchored N-Heterocyclic Carbene Rotor,
Nano Lett. 2020, 20, 5922-5928.
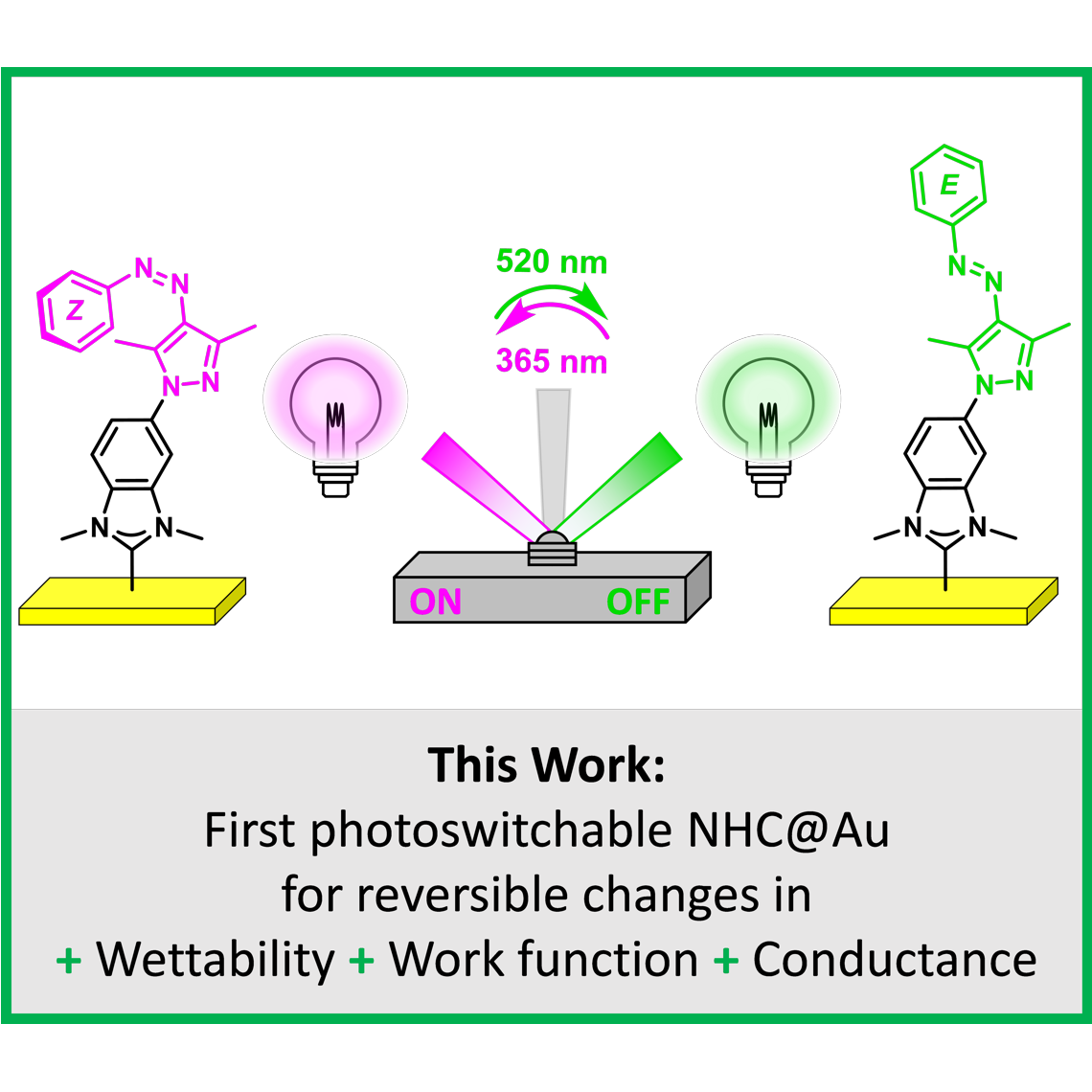
D. T. Nguyen,§ M. Freitag,§ C. Gutheil, K. Sotthewes, B. J. Tyler, M. Böckmann, M. Das, F. Schlüter, N. L. Doltsinis, H. F. Arlinghaus, B. J. Ravoo,* F. Glorius,*
An Arylazopyrazole Based N-Heterocyclic Carbene as Photoswitch on Gold Surfaces: Light-Switchable Wettability, Work Function and Conductance,
Angew. Chem. Int. Ed. 2020,59, 13651-13656; Angew. Chem. 2020,132, 13754-13759.
§ These authors contributed equally.
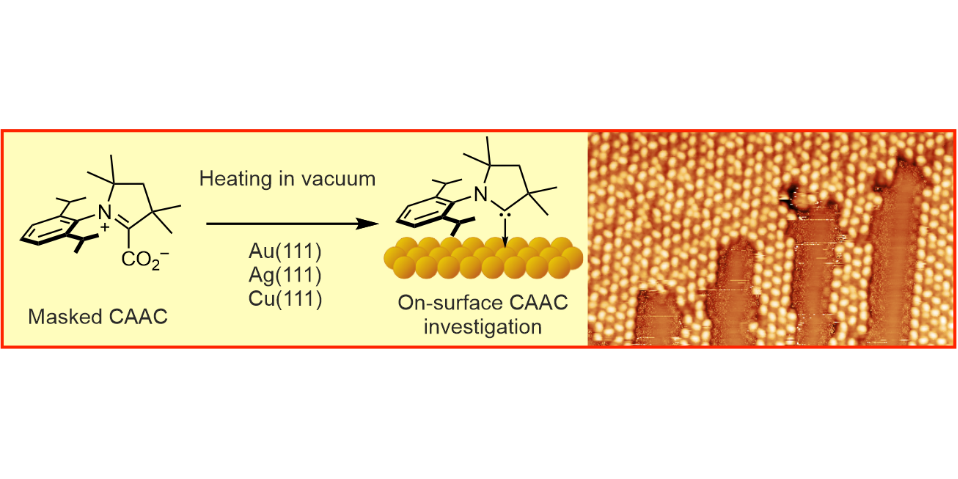
A. Bakker,§ M. Freitag,§ E. Kolodzeiski, P. Bellotti, A. Timmer, J. Ren, B. Schulze Lammers, D. Moock, H. W. Roesky, H. Mönig, S. Amirjalayer, H. Fuchs,* F. Glorius,*
An Electron-rich Cyclic (Alkyl)(Amino) Carbene on Au(111), Ag(111) and Cu(111) Surfaces,
Angew. Chem. Int. Ed. 2020, 59, 13643-13646; Angew. Chem. 2020, 132, 13745-13749.
§ These authors contributed equally.
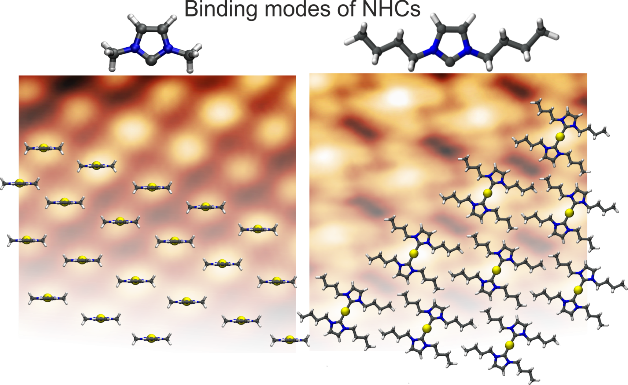
A. Bakker, A. Timmer, E. Kolodzeiski, M. Freitag, H. Y. Gao, H. Mönig, S. Amirjalayer,* F. Glorius,* H. Fuchs,*
Elucidating the binding modes of N-heterocyclic carbenes on a gold surface,
J. Am. Chem. Soc. 2018, 140, 11889-11892.
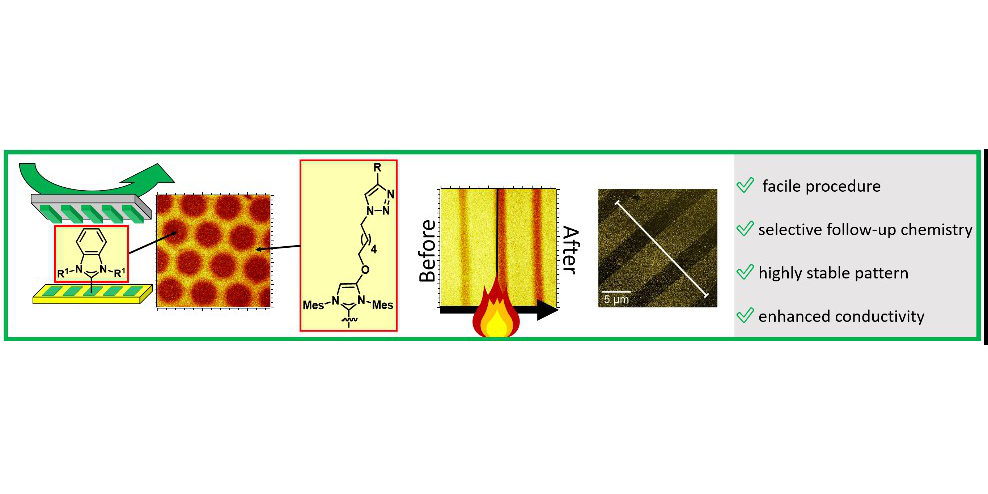
D. T. Nguyen,§ M. Freitag,§ M. Körsgen, S. Lamping, A. Rühling, A. H. Schäfer, M. H. Siekman, H. F. Arlinghaus, W. G. van der Wiel, F. Glorius,* B. J. Ravoo,*
Versatile Micropatterns of N-Heterocyclic Carbenes on Gold Surfaces: Increased Thermal and Pattern Stability with Enhanced Conductivity,
Angew. Chem. Int. Ed. 2018, 57, 11465-11469; Angew. Chem. 2018, 130, 11637-11641.
§ Both authors contributed equally.
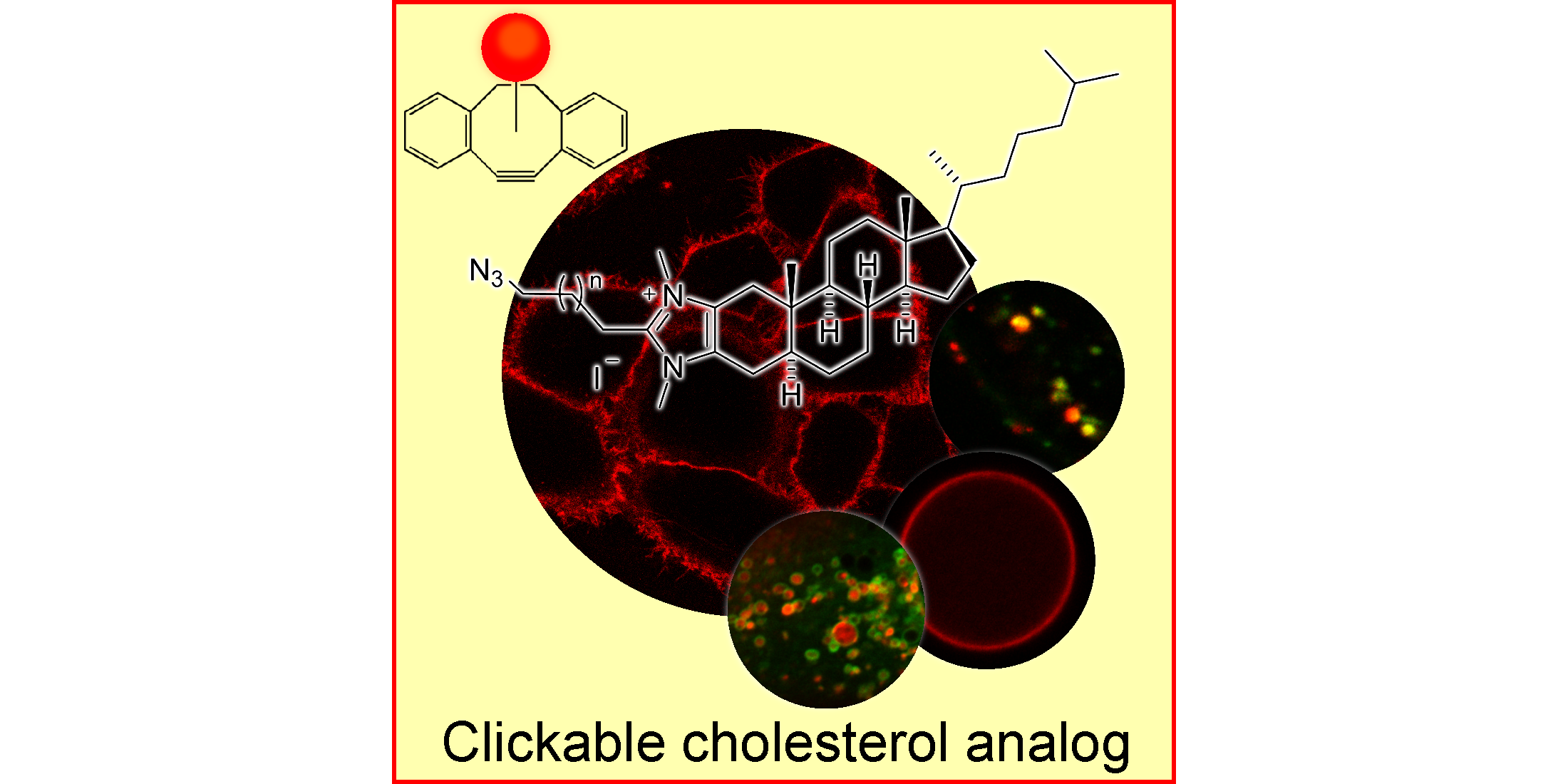
L. Rakers, D. Grill, A. L. L. Matos, S. Wulff, D. Wang, J. Börgel, M. Körsgen, H. F. Arlinghaus, H.-J. Galla,* V. Gerke,* F. Glorius,*
Addressable Cholesterol Analogs for Live Imaging of Cellular Membranes,
Cell Chem. Biol. 2018, 25, 952-961.e12.
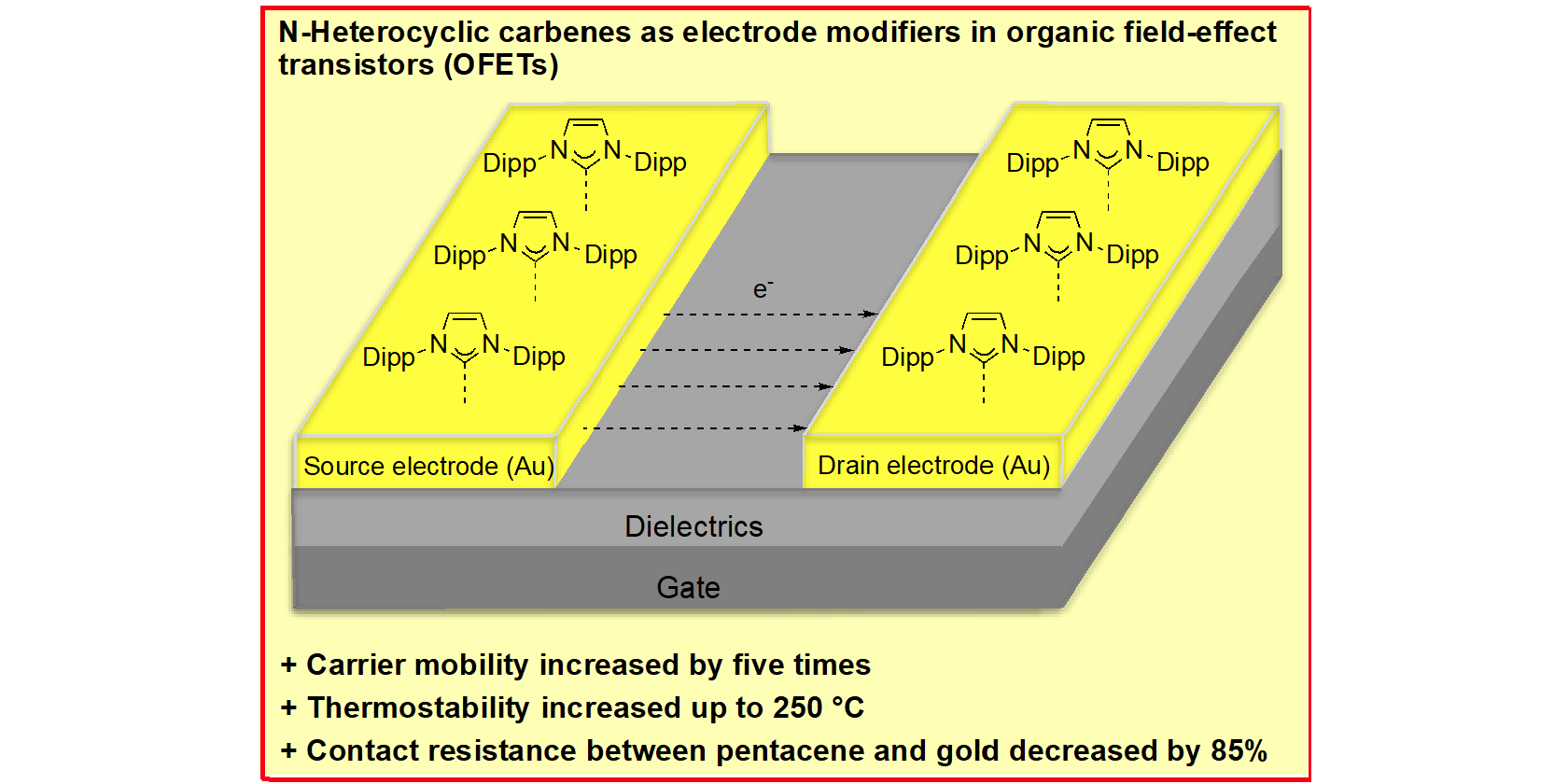
A. Lv, M. Freitag, K. M. Chepiga, A. H. Schäfer, F Glorius,* L. Chi,*
N-Heterocyclic Carbene-Treated Gold Surfaces in Pentacene Organic Field-Effect Transistors: Improved Stability and Contact at the Interface,
Angew. Chem. Int. Ed. 2018, 57, 4792-4796; Angew. Chem. 2018, 130, 4883-4887.
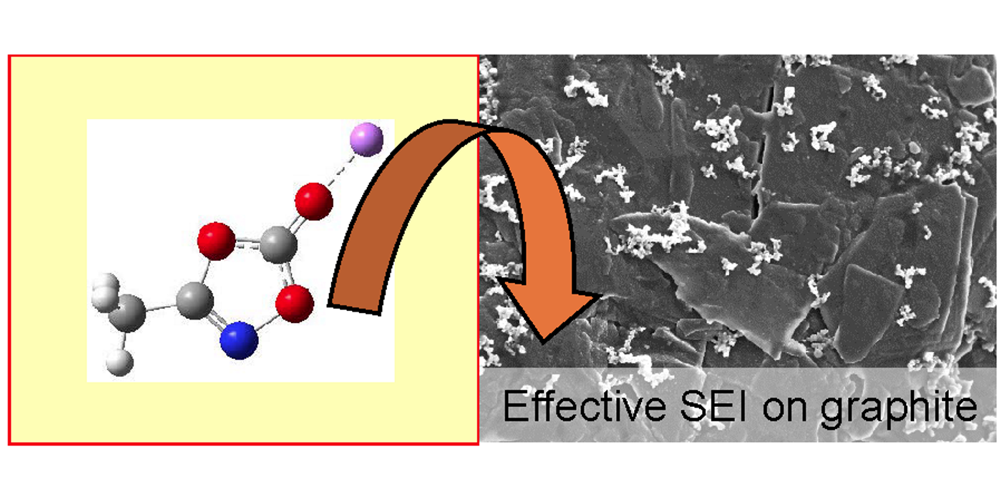
S. Röser, A. Lerchen, L. Ibing, X. Cao, J. Kasnatscheew, F. Glorius,* M. Winter,* R. Wagner,*
Highly effective solid electrolyte interphase (SEI)-forming electrolyte additive enabling high voltage lithium ion batteries,
Chem. Mater. 2017, 29, 7733-7739.
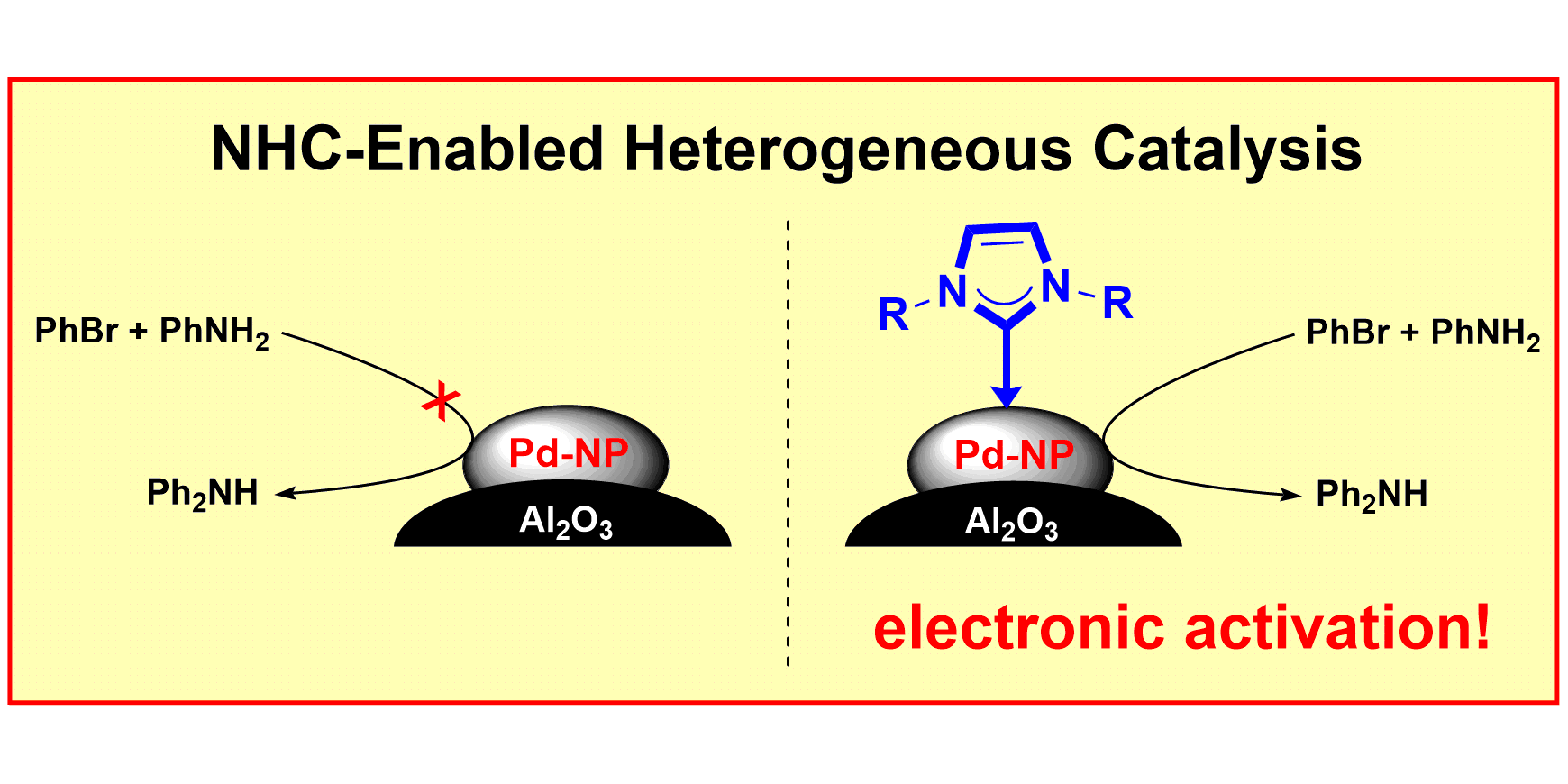
J. B. Ernst, C. Schwermann, G.-I. Yokota, M. Tada, S. Muratsugu,* N. L. Doltsinis,* F. Glorius,*
Molecular Adsorbates Switch on Heterogeneous Catalysis: Induction of Reactivity by N-Heterocyclic Carbenes,
J. Am. Chem. Soc. 2017, 139, 9144-9147.
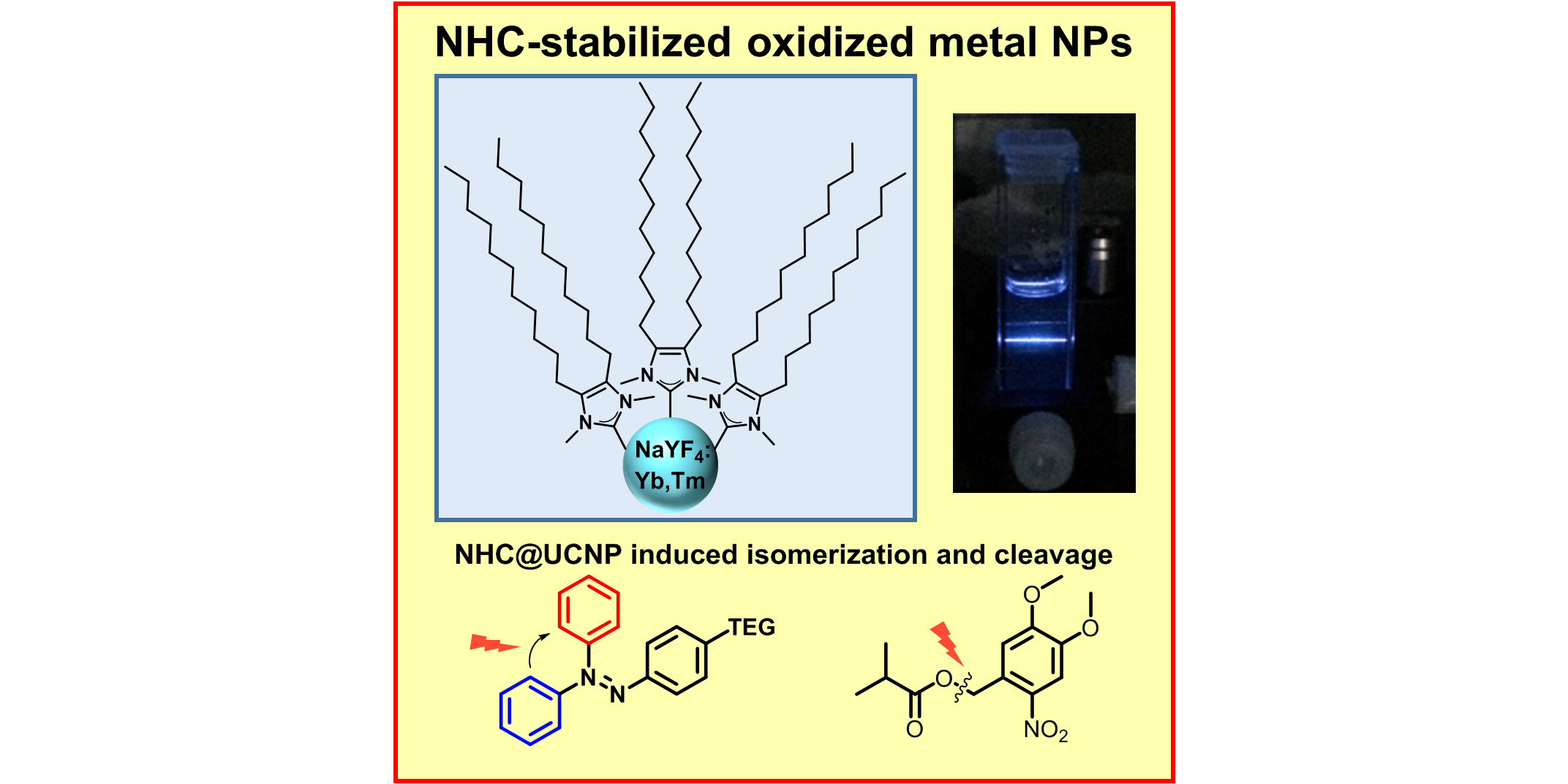
N. Möller§, A. Rühling§, S. Lamping, T. Hellwig, C. Fallnich, B. J. Ravoo,* F. Glorius,*
Stabilization of High Oxidation State Upconversion Nanoparticles by N-Heterocyclic Carbenes (NHCs),
Angew. Chem. Int. Ed. 2017, 56, 4356-4360; Angew. Chem. 2017, 129, 4421-4425.
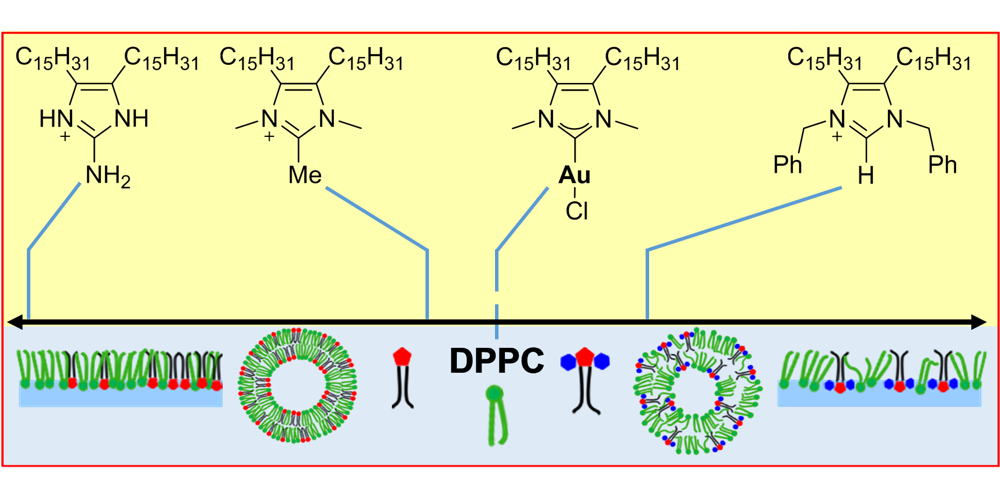
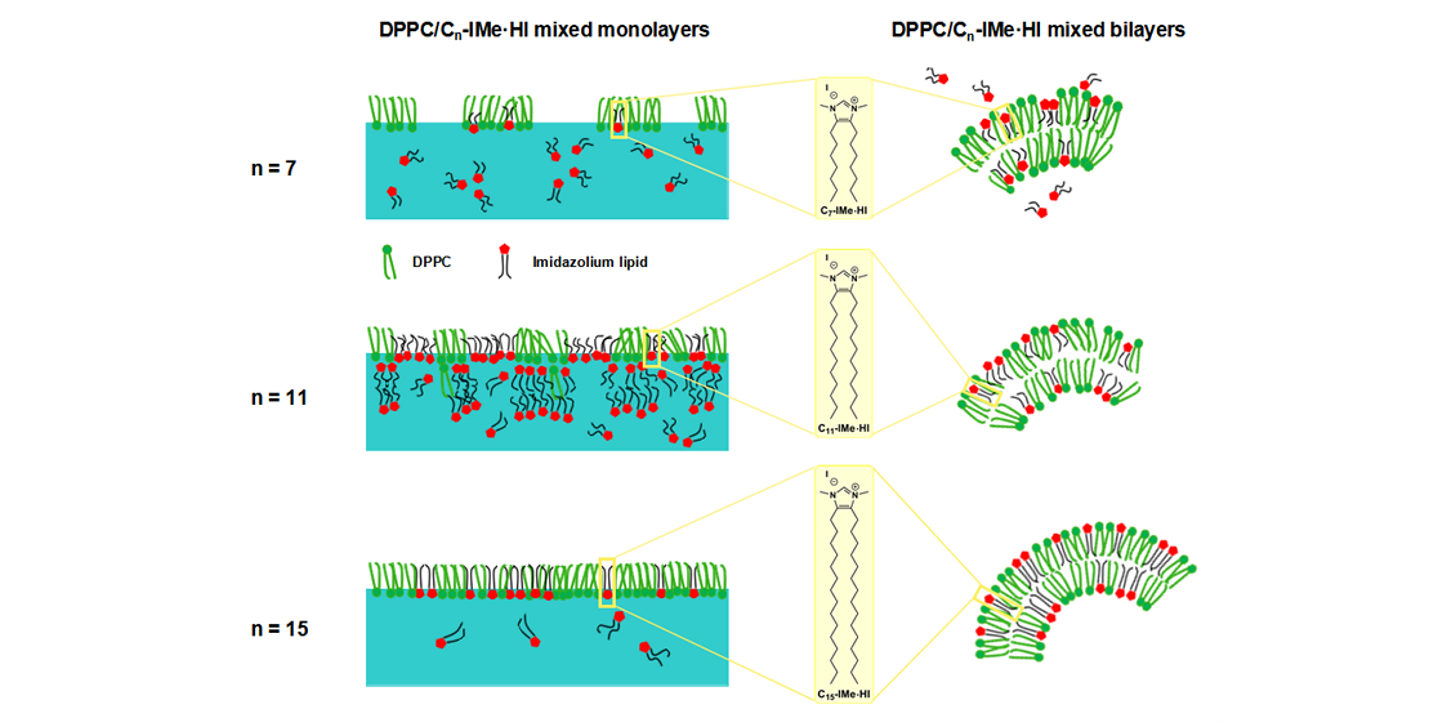
A. Rühling, D. Wang, J. B. Ernst, S. Wulff, R. Honeker, C. Richter, A. Ferry, H.-J. Galla,* F. Glorius,*
Influence of the Headgroup of Azolium-Based Lipids on Their Biophysical Properties and Cytotoxicity,
Chem. Eur. J. 2017, 23, 5920-5924.
P. Drücker, A. Rühling, D. Grill, D. Wang, A. Draeger, V. Gerke, F. Glorius,* H.-J. Galla,*
Imidazolium Salts Mimicking the Structure of Natural Lipids Exploit Remarkable Properties Forming Lamellar Phases and Giant Vesicles,
Langmuir 2017, 33, 1333-1342.
G. Wang, A. Rühling, S. Amirjalayer, M. Knor, J. B. Ernst, C. Richter, H.-J. Gao, A. Timmer, H.-Y. Gao, N. L. Doltsinis, F. Glorius,* H. Fuchs,*
Ballbot-type motion of N-heterocyclic carbenes on gold surfaces,
Nature Chem. 2017, 9, 152-156.
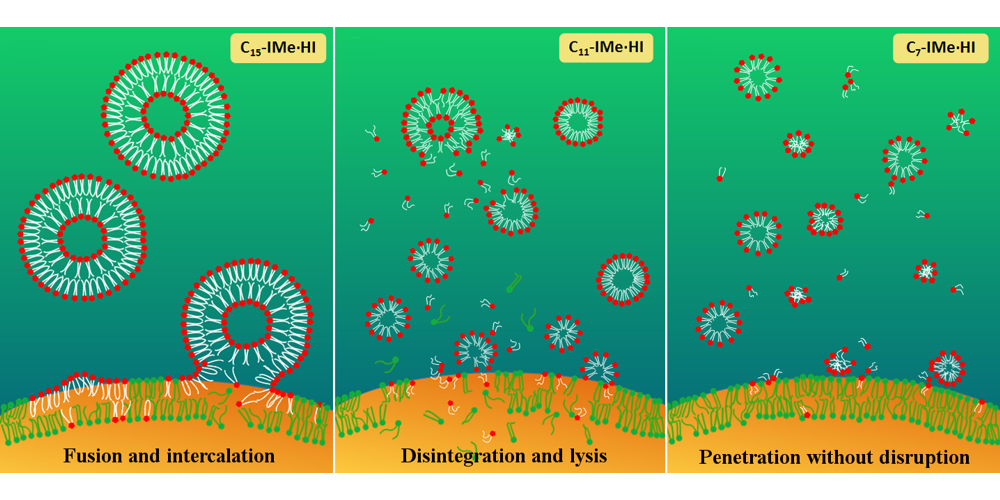
D. Wang, D. H. de Jong, A. Rühling, V. Lesch, K. Shimizu, S. Wulff, A. Heuer,* F. Glorius,* H.-J. Galla,*
Imidazolium-Based Lipid Analogues and Their Interaction with Phosphatidylcholine Membranes,
Langmuir 2016, 32, 12579–12592.
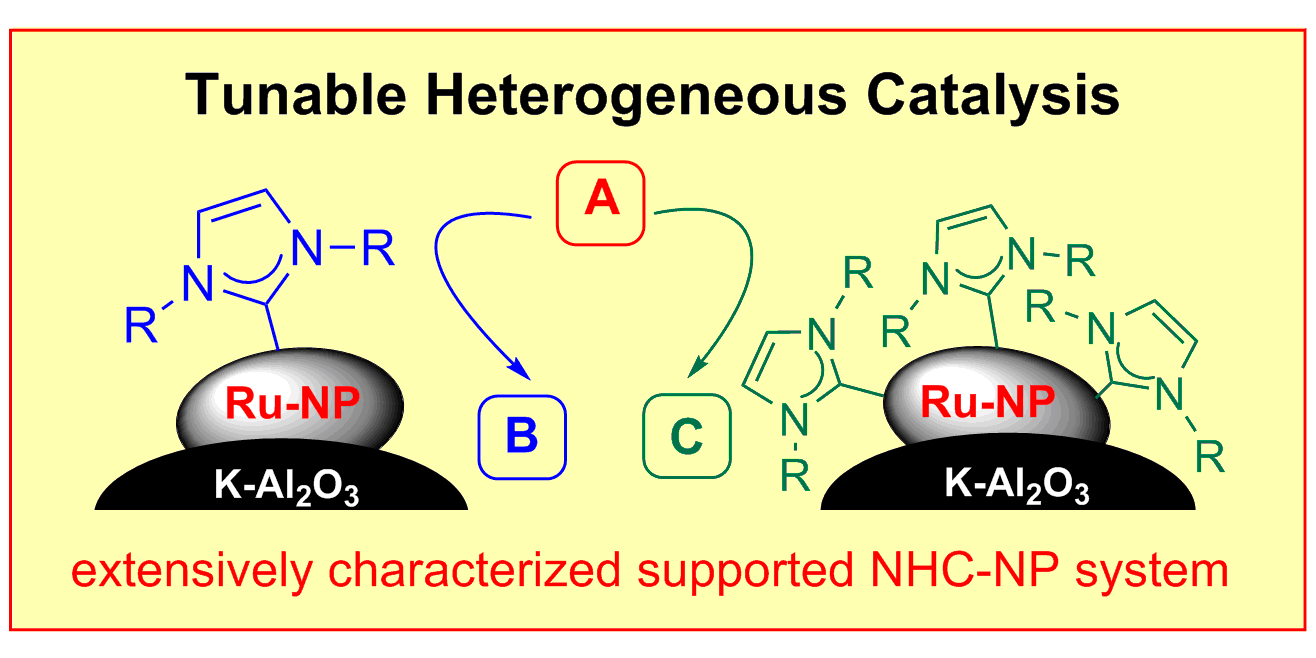
J. B. Ernst, S. Muratsugu,* F. Wang, M. Tada, F. Glorius,*
Tunable Heterogeneous Catalysis – N-Heterocyclic Carbenes as Ligands for Supported Heterogeneous Ru/K-Al2O3 Catalysts to Tune Reactivity and Selectivity,
J. Am. Chem. Soc. 2016, 138, 10718-10721.
C. Richter§, K. Schaepe§, F. Glorius,* B. J. Ravoo,*
Tailor-made N-Heterocyclic Carbenes for Nanoparticle Stabilization,
Chem. Commun. 2014, 50, 3204-3207.
§ Both authors contributed equally.