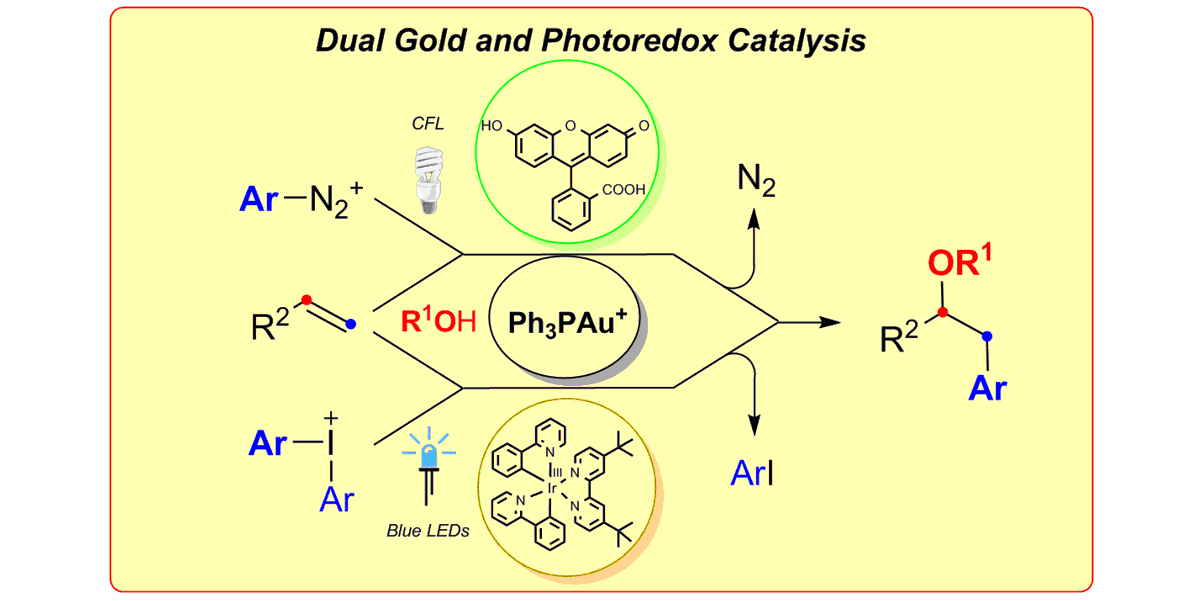
Photocatalysis
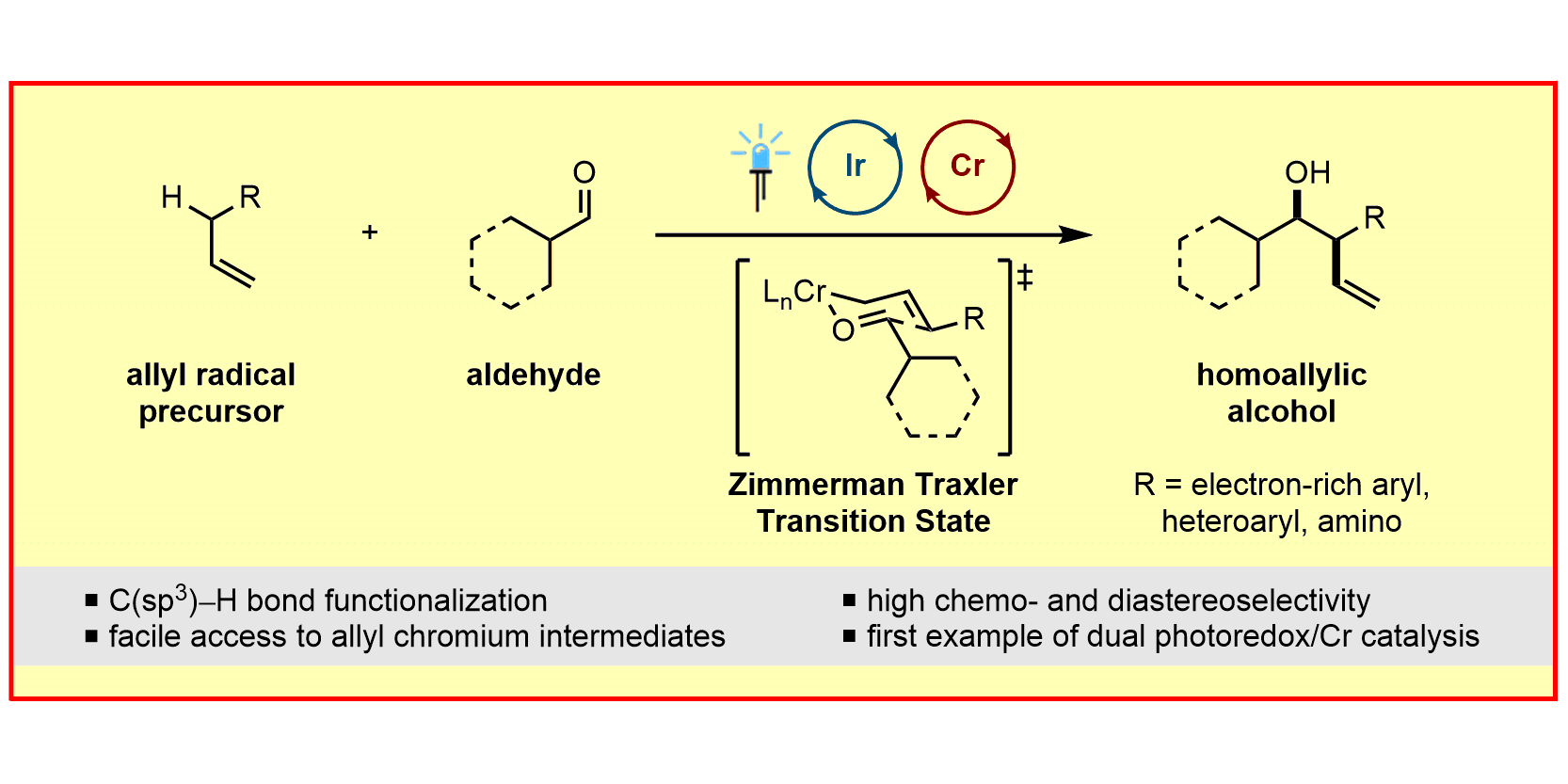
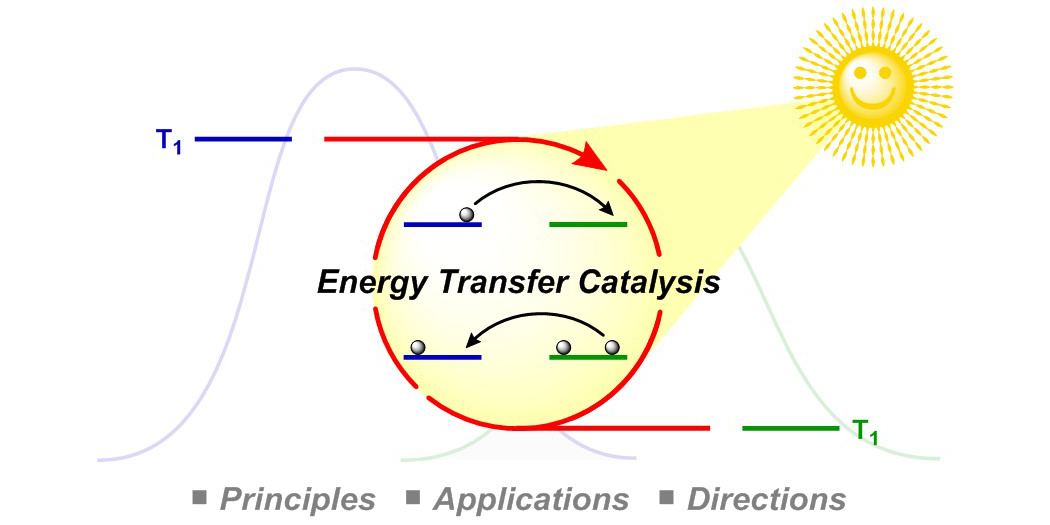
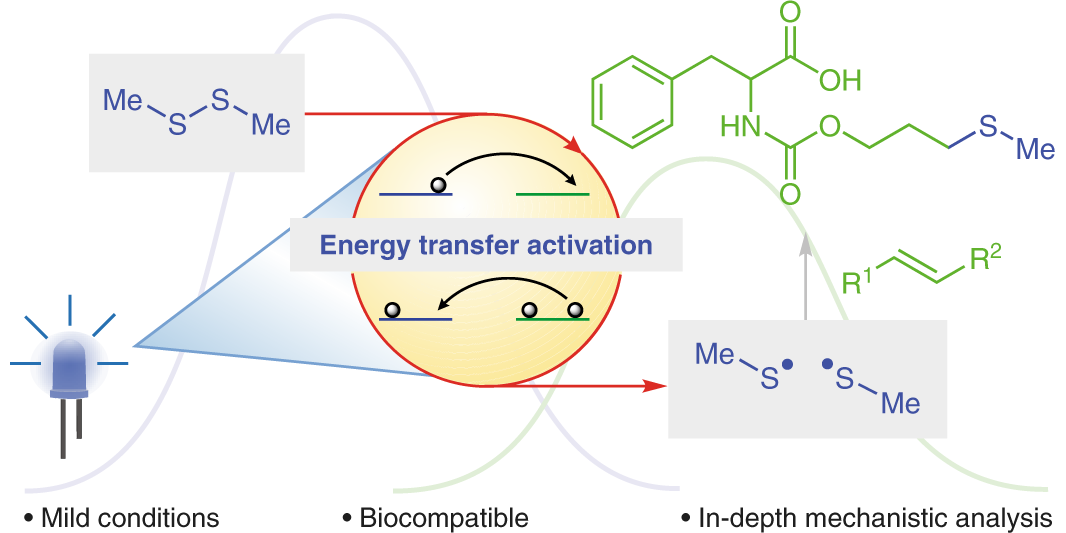
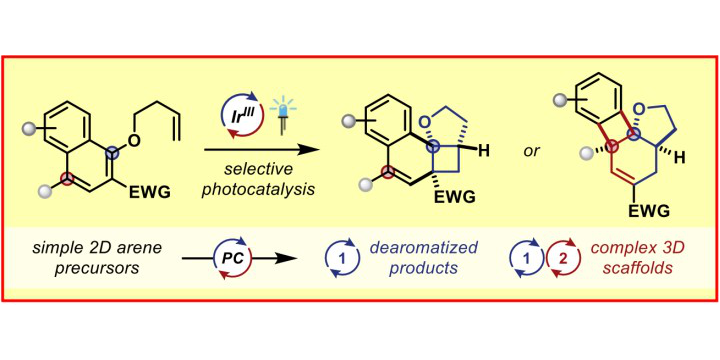
Photoredox catalysis is a modern tool to generate highly reactive radical species under remarkably mild conditions and thus opens up completely new opportunities for synthetic chemists. The photocatalyst harnesses the energy of light to accelerate chemical reactions either via electron-transfer processes or via direct energy transfer to an organic molecule. In particular, the combination of photoredox catalysis with transition metal catalysis enables the rapid build-up of molecular complexity from readily available starting materials. In that regard, our group was the first to develop photoredox/Au[1] and very recently photoredox/Cr[2] dual-catalytic systems. The later addressed major limitations of the Nozaki-Hiyama-Kishi reaction, e.g. the need for prefunctionalized substrates, stoichiometric quantities of Lewis acid additives and reductants. In contrast, energy-transfer catalysis can be used to facilitate excited state reactivity without the need for harsh UV irradiation. Utilizing this concept, our group developed various dearomatization processes to convert simple 2D arenes to highly complex, 3D frameworks.[3,4] Additionally, our group focuses on the cleavage of σ-bonds using energy-transfer catalysis to enable novel transformations.[5]
[1] B. Sahoo, M. N. Hopkinson, F. Glorius, J. Am. Chem. Soc. 2013, 135, 5505. [2] J. L. Schwarz, F. Schäfers, A. Tlahuext-Aca, L. Lückemeier, F. Glorius, J. Am. Chem. Soc. 2018, 140, 12705. [3] M. J. James, J. L. Schwarz, F. Strieth-Kalthoff, B. Wibbeling, F. Glorius, J. Am. Chem. Soc. 2018, 140, 8624. [4] F. Strieth-Kalthoff, C. Henkel, M. Teders, A. Kahnt, W. Knolle, A. Gómez-Suárez, K. Dirian, W. Alex, K. Bergander, C. G. Daniliuc, B. Abel, D. M. Guldi, F. Glorius, Chem 2019, 5, 2183. [5] T. Patra, S. Mukherjee, J. Ma, F. Strieth-Kalthoff, F. Glorius, Angew. Chem. Int. Ed. 2019, 58, 10514.
Please click on the graphical abstracts to come to the original publication
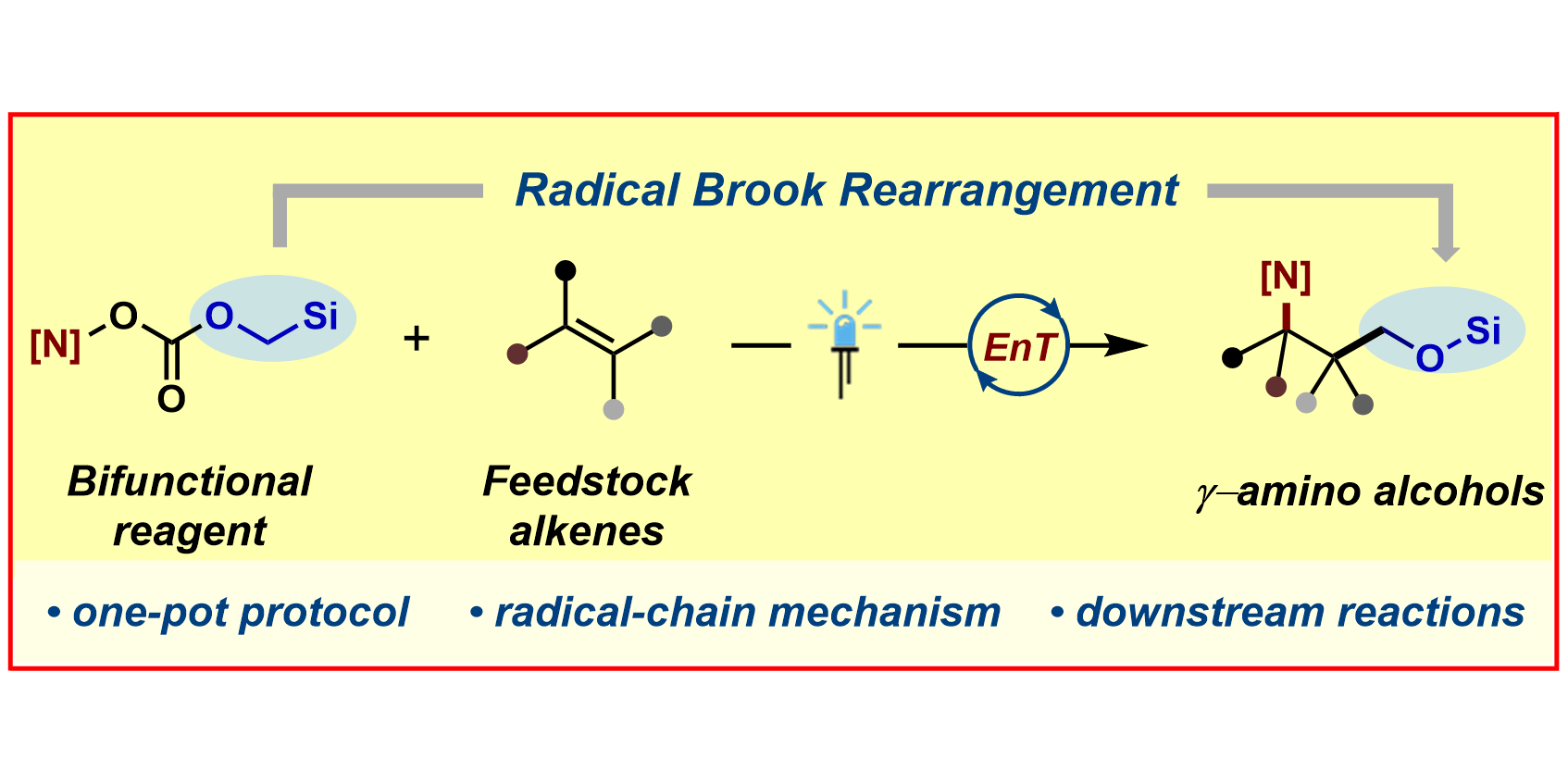
R. Laskar,§ S. Dutta,§ J. C. Spies, P. Mukherjee, A. Renteria-Gomez, R. E. Thielemann, C. G. Daniliuc, O. Gutierrez,* F. Glorius,*
γ-Amino Alcohols via Energy Transfer Enabled Brook Rearrangement,
J. Am. Chem. Soc. 2024, 146, 10899-10907.
§ These authors contributed equally.
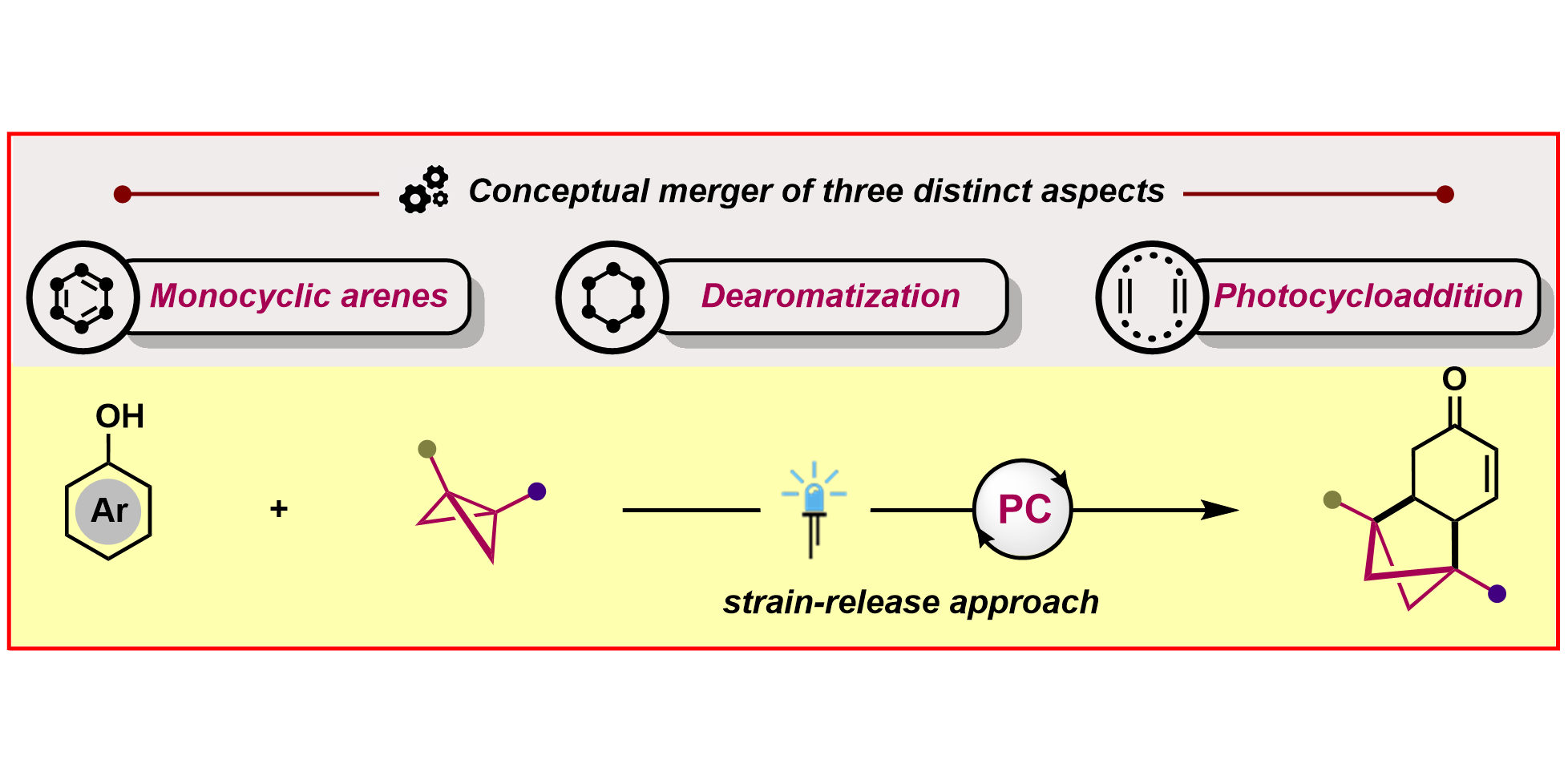
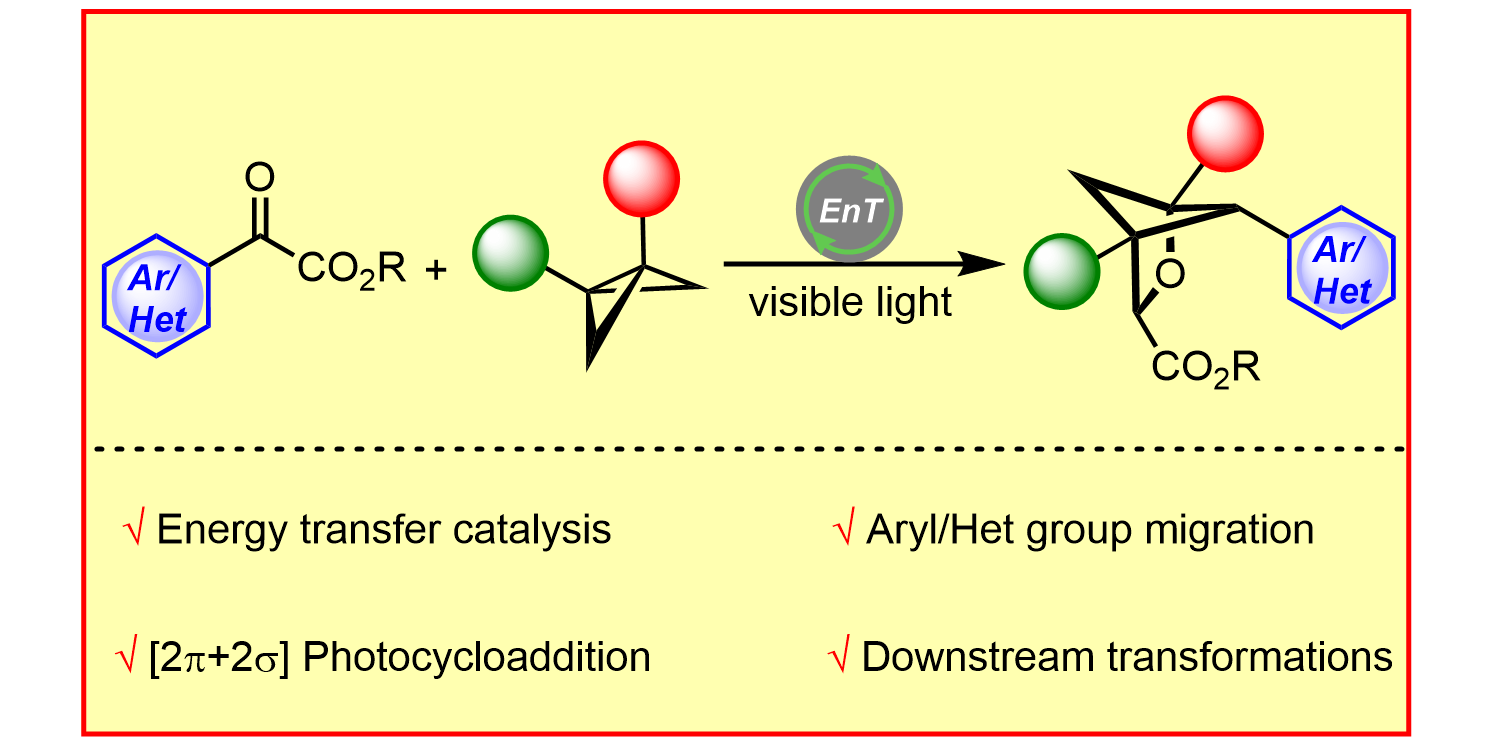
S. Dutta, Y.-L. Lu,§ J. E. Erchinger,§ H. Shao, E. Studer, F. Schäfer, H. Wang, D. Rana, C. G. Daniliuc, K. N. Houk,* F. Glorius,*
Double Strain-Release [2π+2σ]-Photocycloaddition,
J. Am. Chem. Soc. 2024, 146, 5232-5241.
§ These authors contributed equally.
S. Dutta,§ D. Lee,§ K. Ozols,§ C. Daniliuc, R. Shintani, F. Glorius,
Photoredox-Enabled Dearomative [2π+2σ] Cycloaddition of Phenols,
J. Am. Chem. Soc. 2024, 146, 2789-2797.
§ These authors contributed equally.
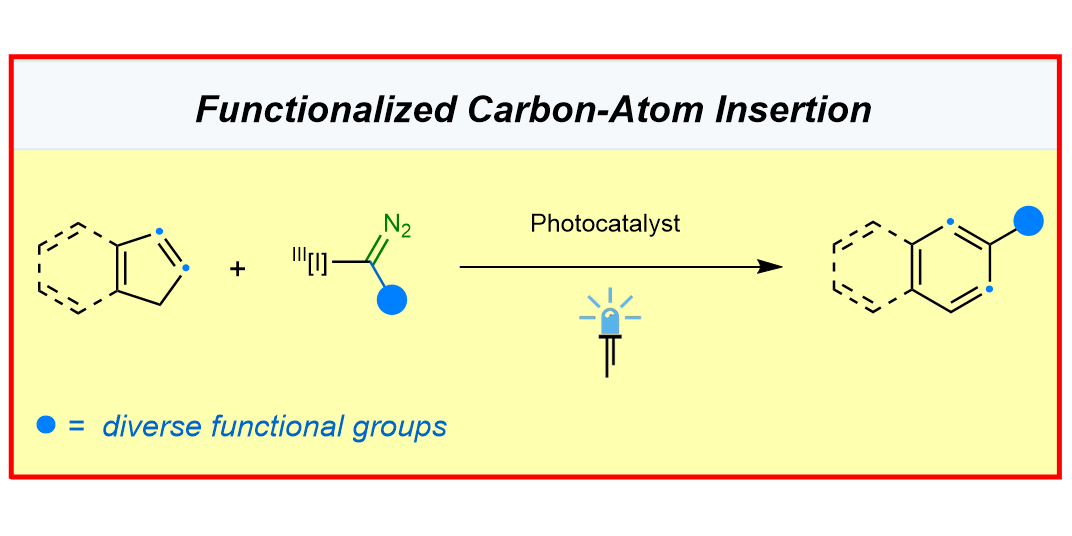
F.-P. Wu, C. C. Chintawar, R. Lalisse, P. Mukherjee, S. Dutta, J. Tyler, C. G. Daniliuc, O. Gutierrez,* F. Glorius,*
Ring expansion of indene by photoredox-enabled functionalized carbon-atom insertion,
Nat. Catal. 2024, 7, 242-251.
S. Dutta, J. E. Erchinger, F. Strieth-Kalthoff, R. Kleinmans, F. Glorius,
Energy transfer photocatalysis: exciting modes of reactivity,
Chem. Soc. Rev. 2024, 53, 1068-1089.
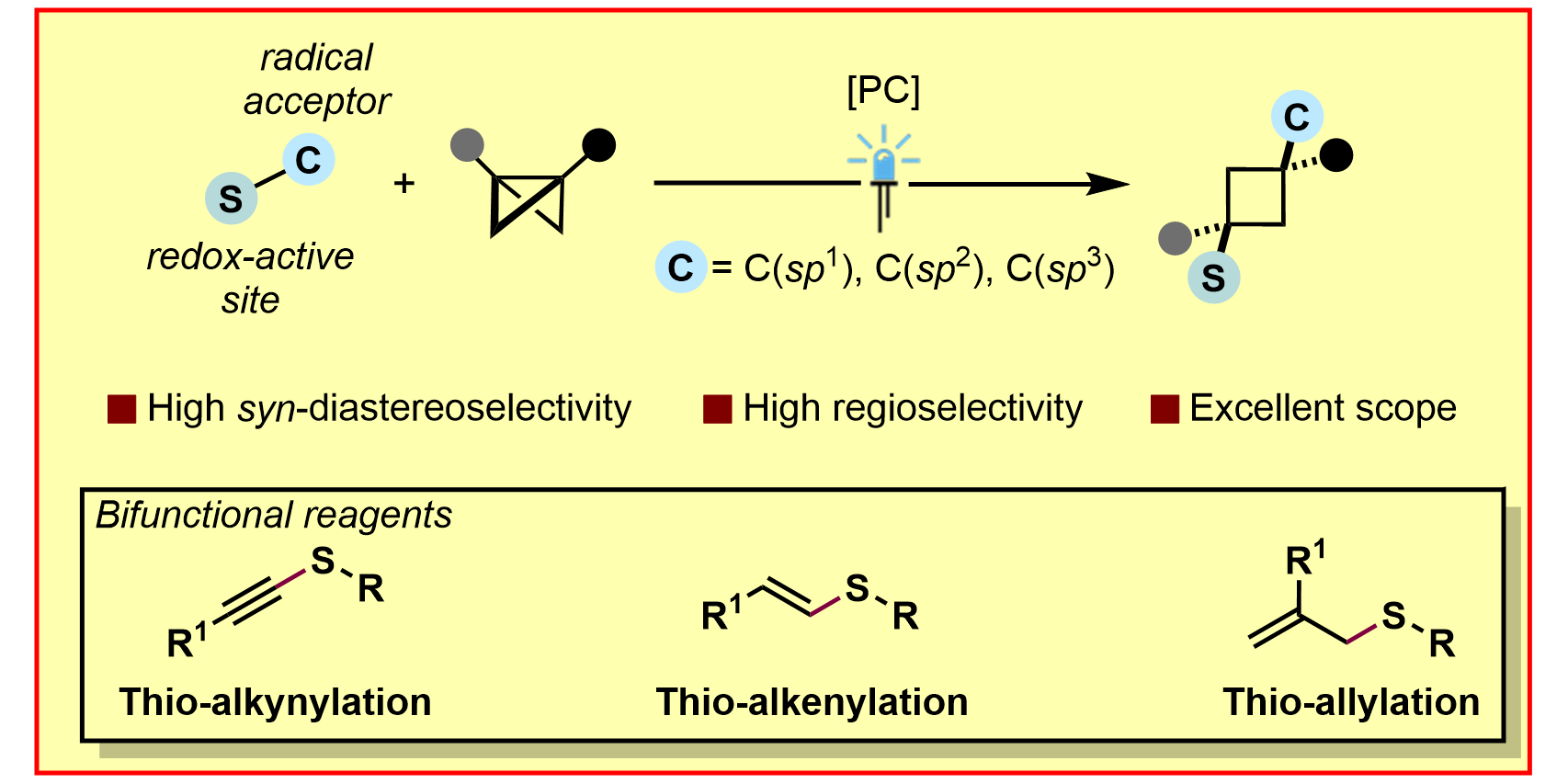
H. Wang,§ J. E. Erchinger,§ M. Lenz,§ S. Dutta, C. G. Daniliuc, F. Glorius,
syn-Selective Difunctionalization of Bicyclobutanes Enabled by Photoredox-Mediated C–S σ-Bond Scission,
J. Am. Chem. Soc. 2023, 145, 23771-23780.
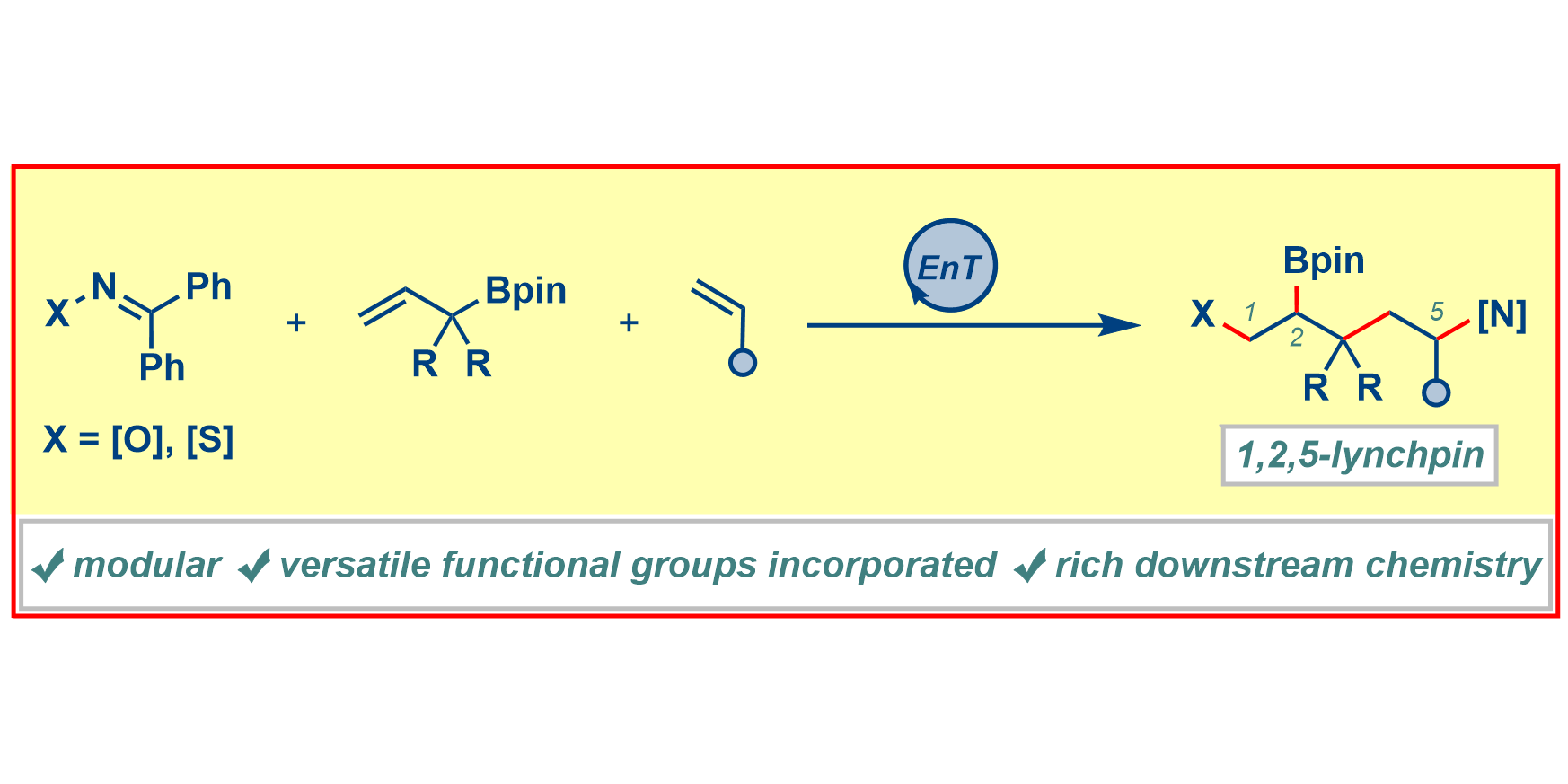
F. Paulus, C. Stein, C. Heusel, T. J. Stoffels, C. G. Daniliuc, F. Glorius,
Three-Component Photochemical 1,2,5-Trifunctionalizations of Alkenes towards Densely Functionalized Lynchpins,
J. Am. Chem. Soc. 2023, 145, 23814-23823.
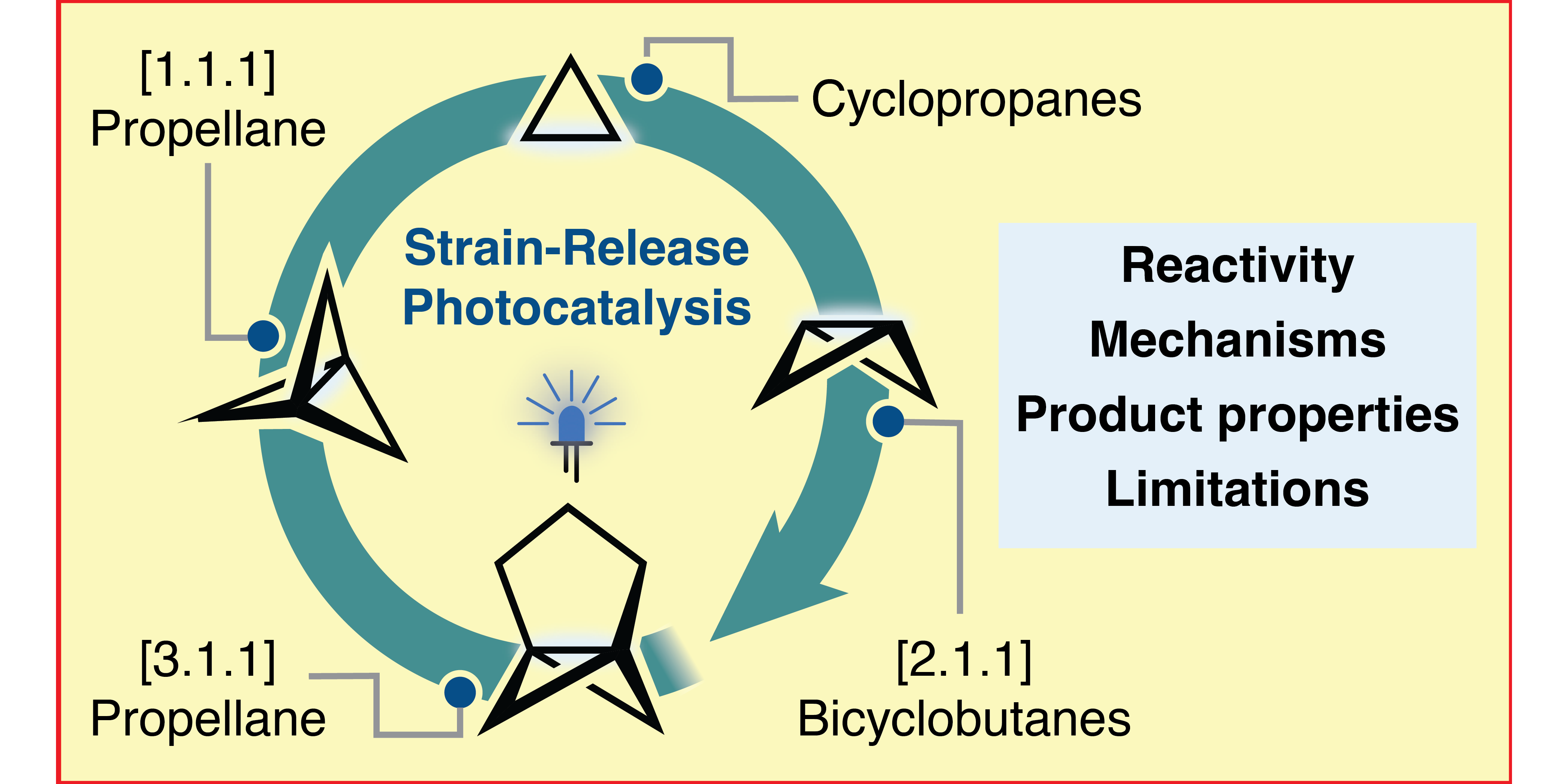
P. Bellotti,* F. Glorius,*
Strain-Release Photocatalysis,
J. Am. Chem. Soc. 2023, 145, 20716-20732.
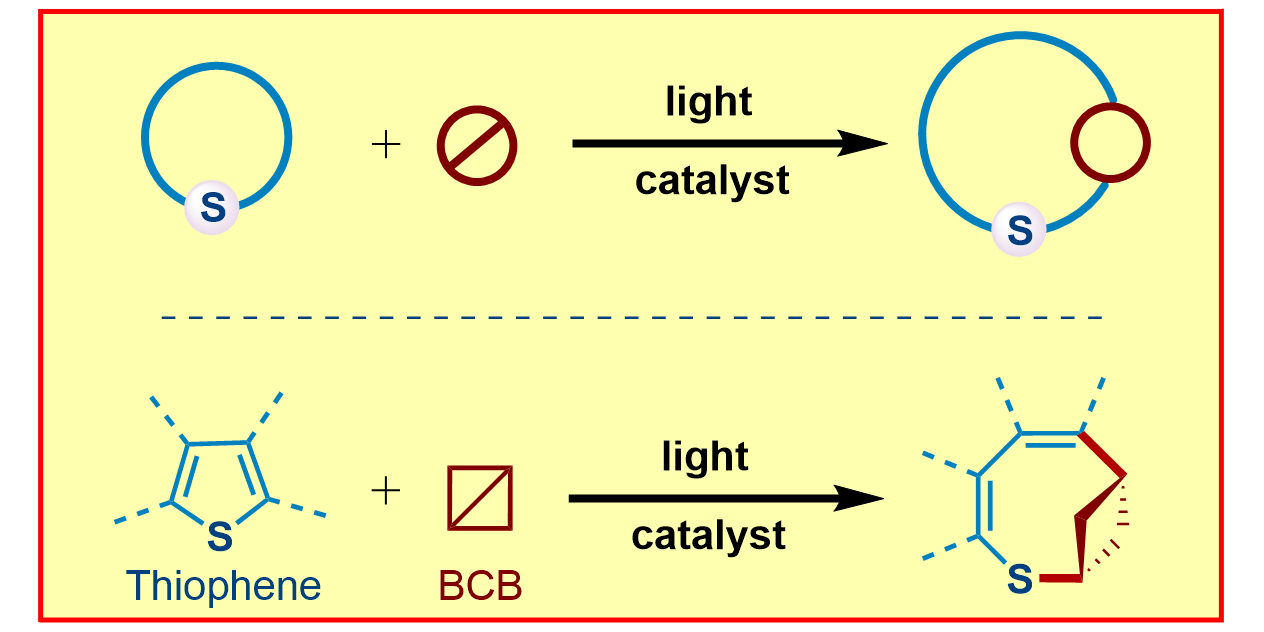
H. Wang, H. Shao,§ A. Das,§ S. Dutta, H. T. Chan, C. Daniliuc, K. N. Houk,* F. Glorius,*
Dearomative ring expansion of thiophenes by bicyclobutane insertion,
Science 2023, 381, 75-81.
§ These authors contributed equally
Free-access electronic reprint
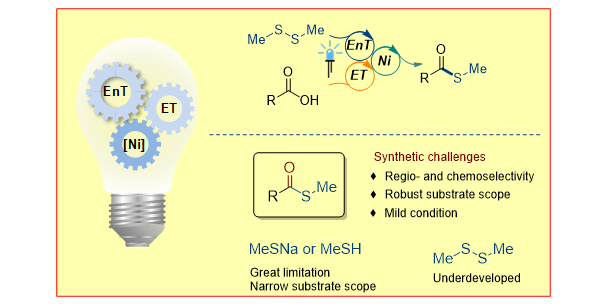
H. Wang, Z. Liu, A. Das, P. Bellotti, S. Megow, F. Temps,* X. Qi,* F. Glorius,*
Radical thioesterification via nickel-catalyzed sensitized electron transfer,
Nature Synth. 2023, 2, 1116-1126.
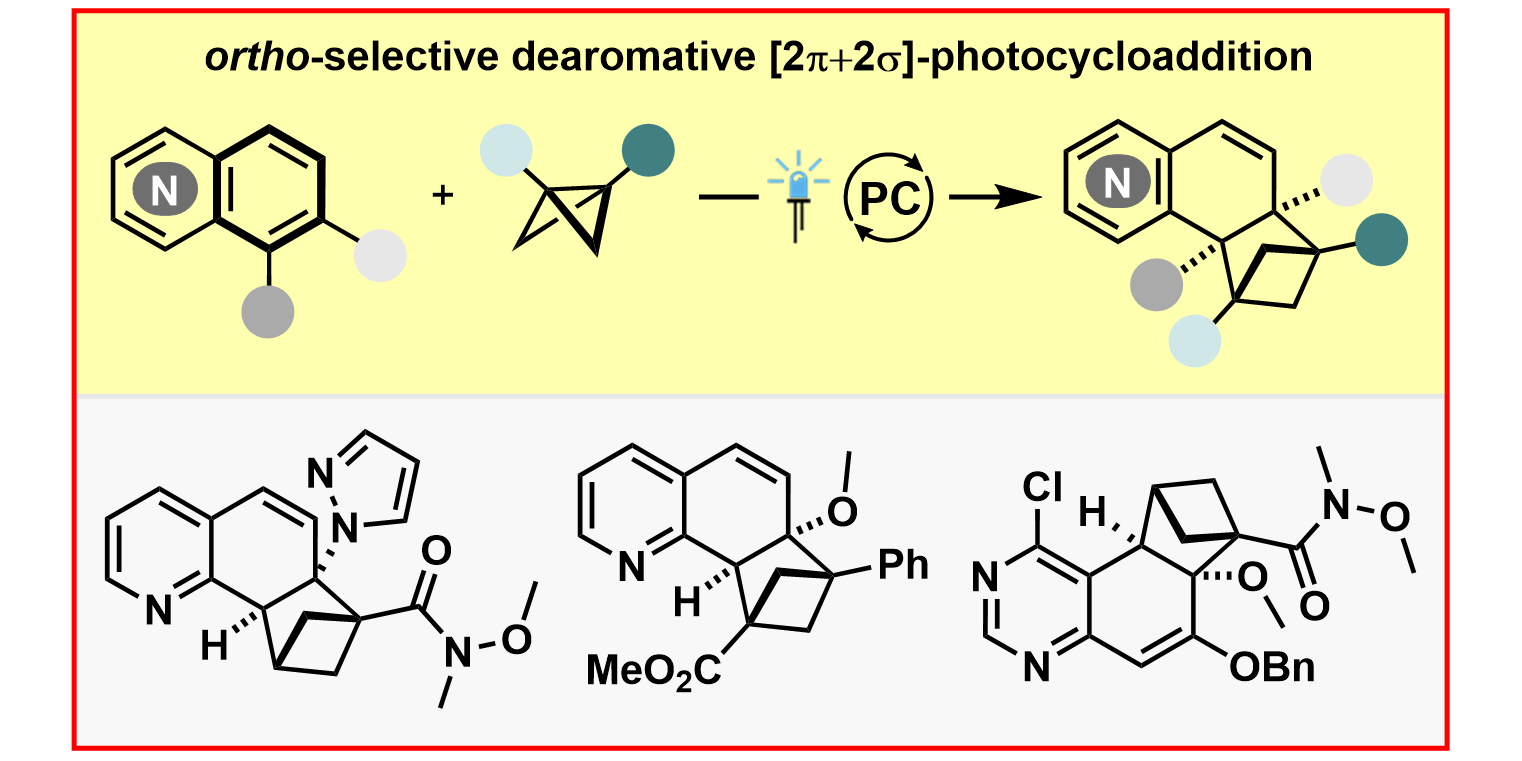
R. Kleinmans,§ S. Dutta,§ K. Ozols, H. Shao, F. Schäfer, R. E. Thielemann, H. T. Chan, C. G. Daniliuc, K. N. Houk,* F. Glorius,*
ortho-Selective Dearomative [2π + 2σ]-Photocycloadditions of Bicyclic Aza-Arenes,
J. Am. Chem. Soc. 2023, 145, 12324-12332.
§ These authors contributed equally
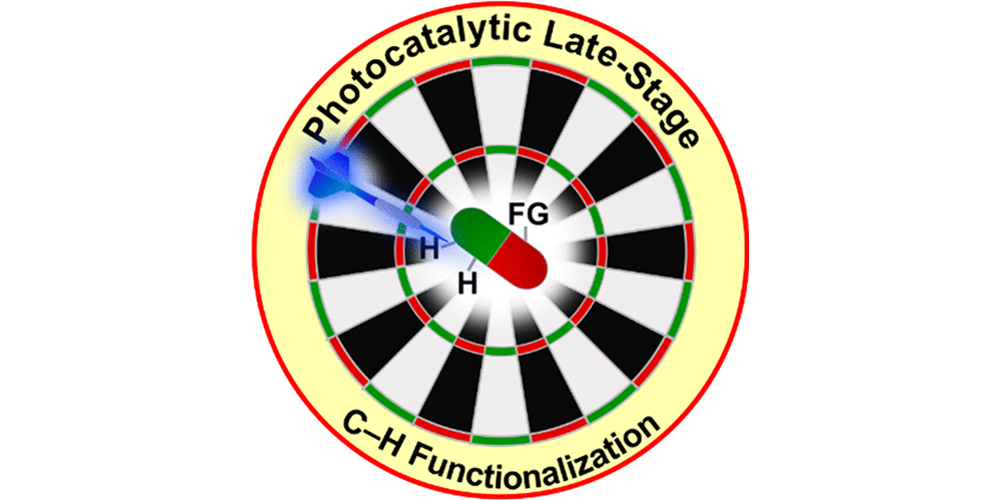
P. Bellotti,§ H.-M. Huang*,§ T. Faber, F. Glorius*,
Photocatalytic Late-Stage C–H Functionalization,
Chem. Rev. 2023, 123, 4237-4352.
§ These authors contributed equally
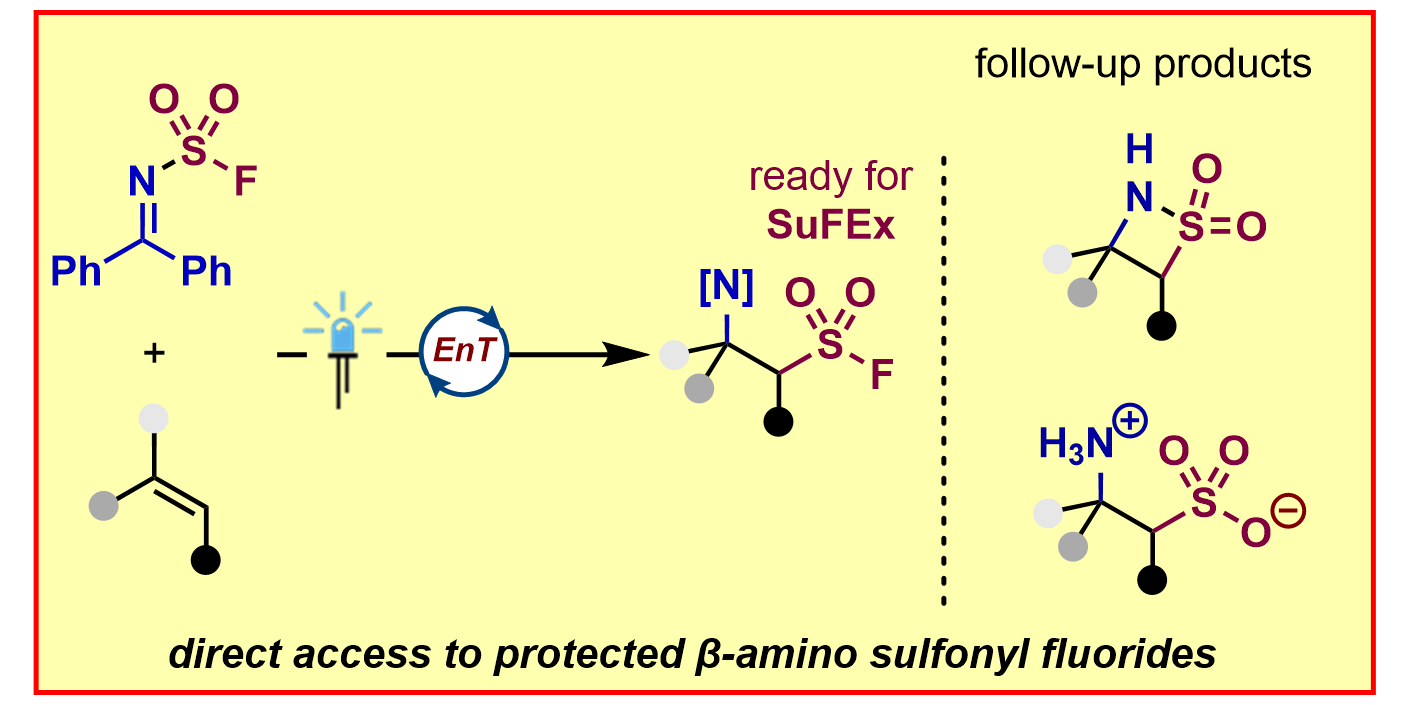
J. E. Erchinger, R. Hoogesteger,§ R. Laskar,§ S. Dutta, C. Hümpel, D. Rana, C. G. Daniliuc, F. Glorius,
EnT-Mediated N–S Bond Homolysis of a Bifunctional Reagent Leading to Aliphatic Sulfonyl Fluorides,
J. Am. Chem. Soc. 2023, 145, 2364-2374.
§ These authors contributed equally
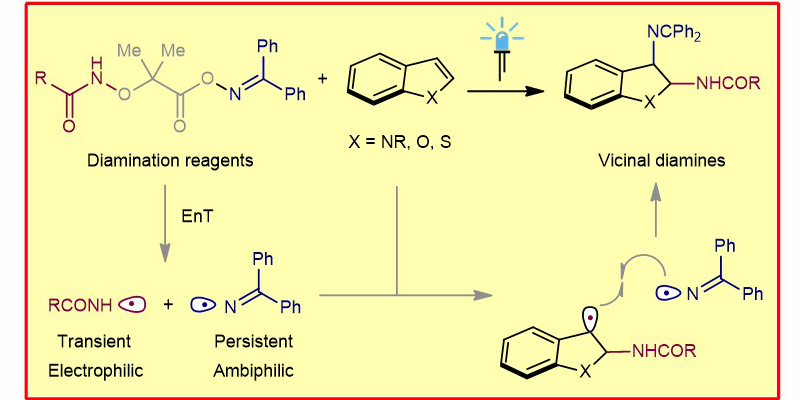
G. Tan, M. Das, R. Kleinmans, F. Katzenburg, C. Daniliuc, F. Glorius,
Energy Transfer-Enabled Unsymmetrical Diamination Using Bifunctional Nitrogen-Radical Precursors,
Nat. Catal. 2022, 5, 1120-1130.
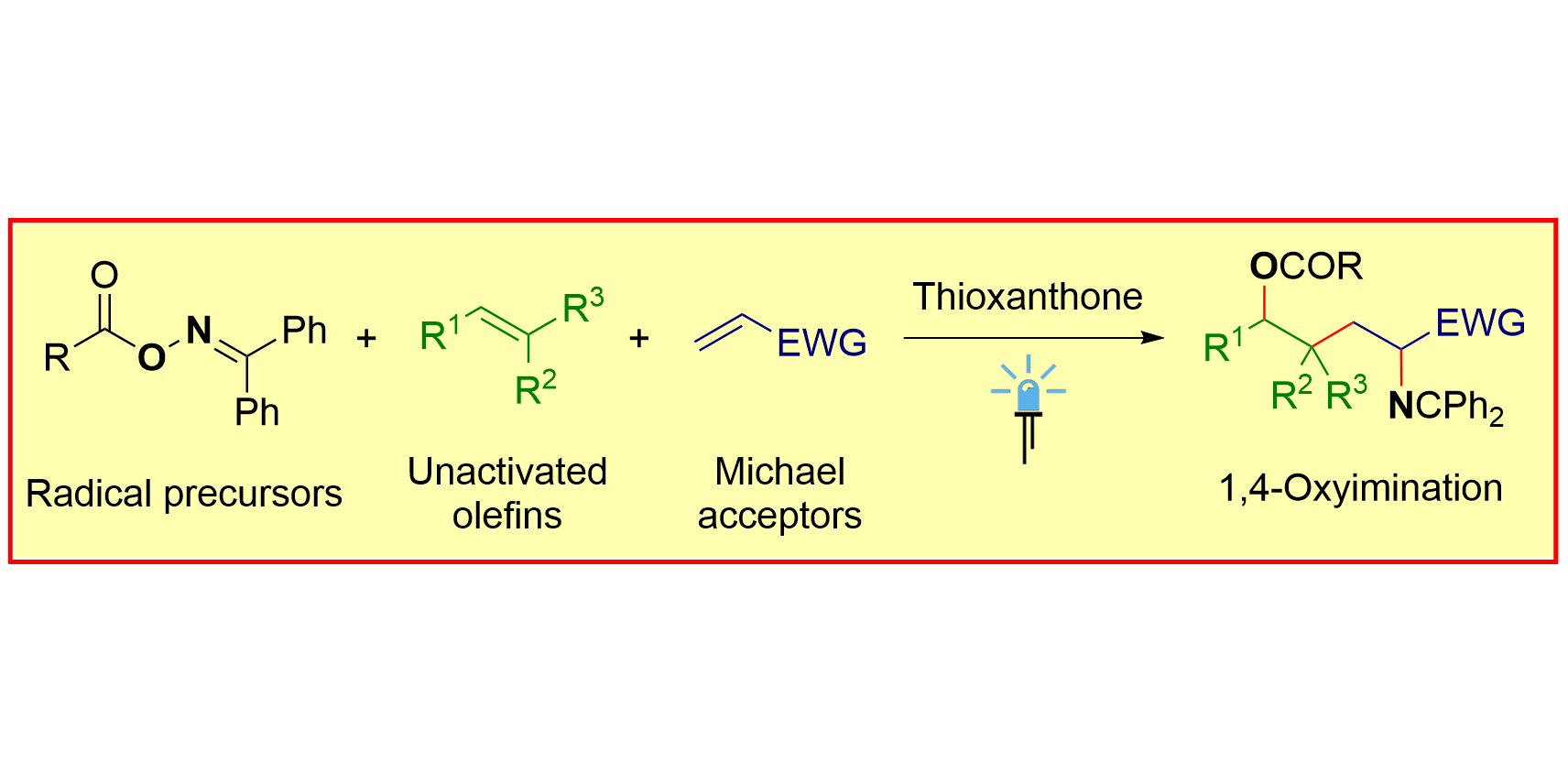
G. Tan, F. Paulus, Á. Rentería-Gómez,§ R. F. Lalisse,§ C. G. Daniliuc, O. Gutierrez*, F. Glorius*,
Highly Selective Radical Relay 1,4-Oxyimination of Two Electronically Differentiated Olefins,
J. Am. Chem. Soc. 2022, 144, 21664-21673.
§ These authors contributed equally
Y. Liang, R. Kleinmans, C. G. Daniliuc, F. Glorius,
Synthesis of Polysubstituted 2-Oxabicyclo[2.1.1]hexanes via Visible-Light-Induced Energy Transfer,
J. Am. Chem. Soc. 2022, 144, 20207-20213.
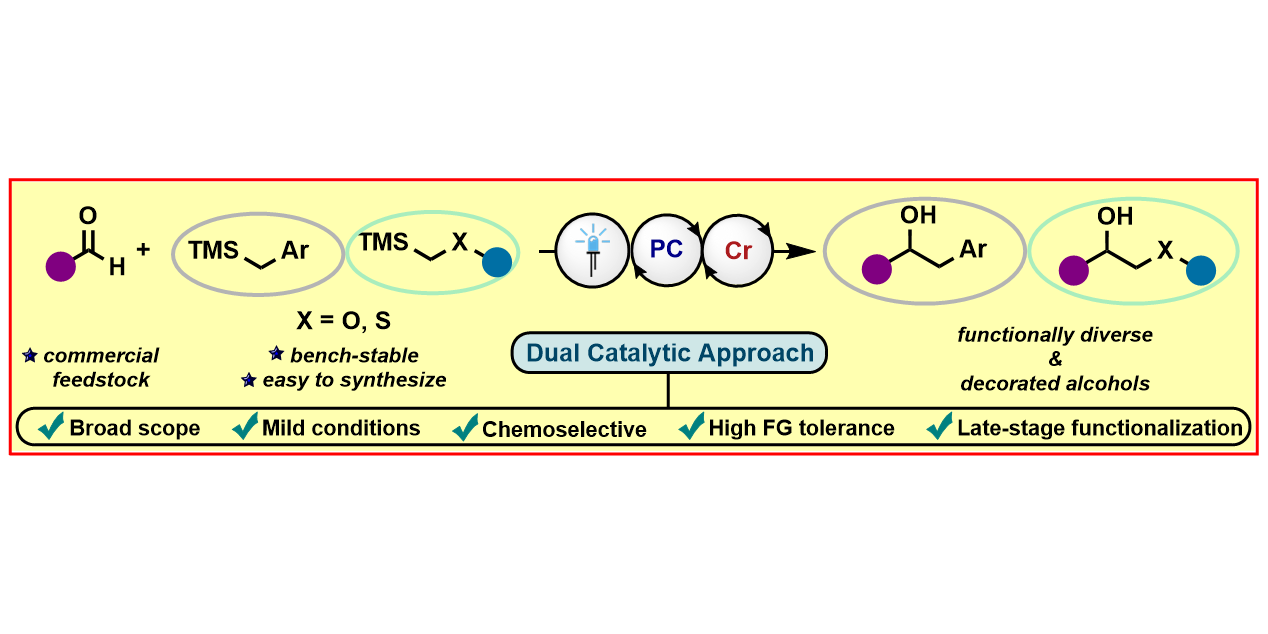
S. Dutta,§ J. E. Erchinger,§ F. Schäfers, A. Das, C. G Daniliuc, F. Glorius,
Chromium/Photoredox Dual-Catalyzed Synthesis of α-Benzylic Alcohols, Isochromanones, 1,2-Oxy Alcohols and 1,2-Thio Alcohols,
Angew. Chem. Int. Ed. 2022, 61 (49), e202212136; Angew. Chem. 2022, 134 (49), e202212136.
§ These authors contributed equally
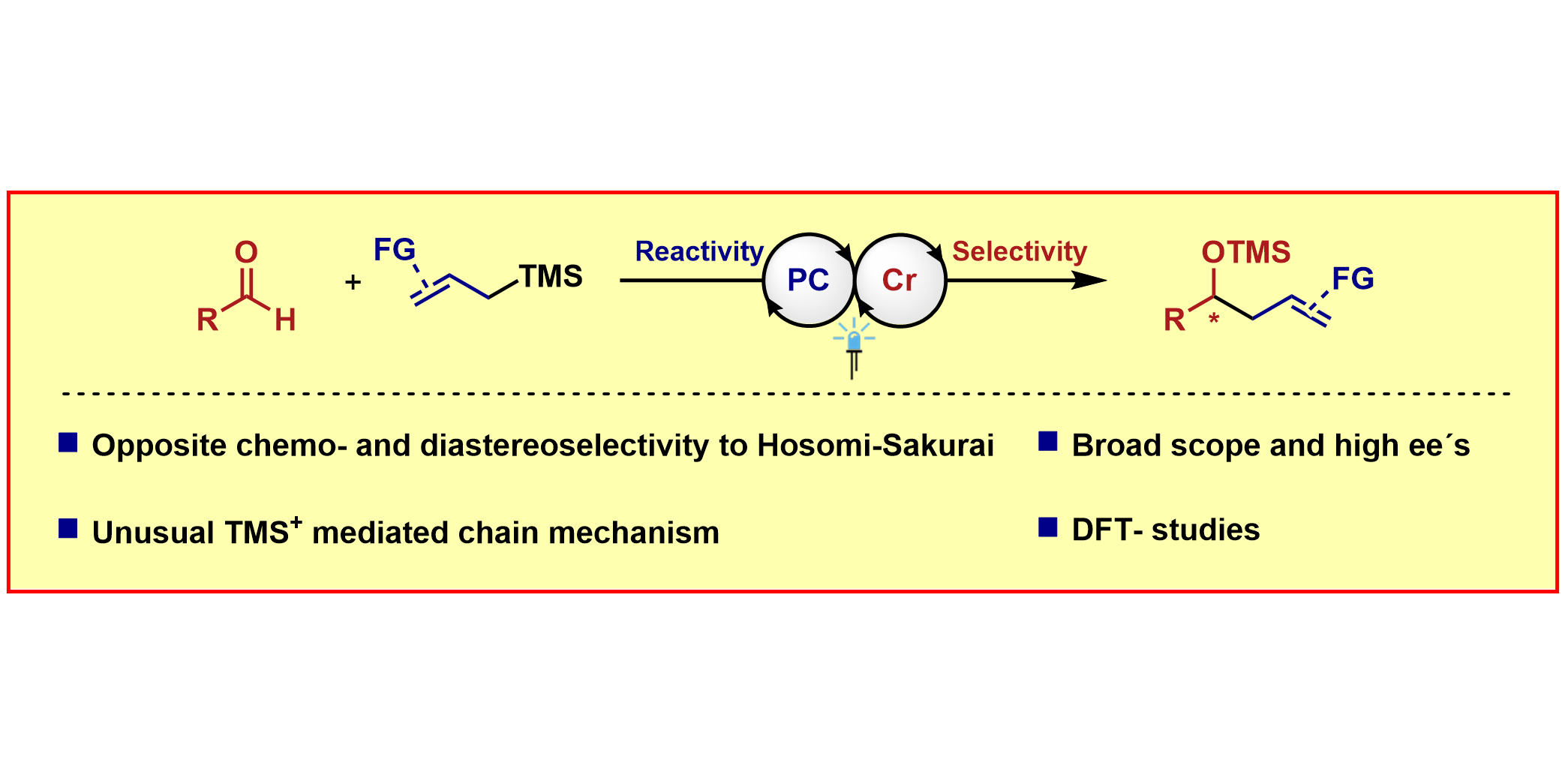
F. Schäfers,§ S. Dutta,§ R. Kleinmans, C. Mück-Lichtenfeld, F. Glorius,
Asymmetric Addition of Allylsilanes to Aldehydes – A Cr/Photoredox Dual Catalytic Approach Complementing the Hosomi–Sakurai Reaction,
ACS Catal. 2022, 12, 12281-12290.
§ These authors contributed equally
R. Guo, S. Adak, P. Bellotti, X. Gao, W. W. Smith, S. N. Le, J. Ma, K. N. Houk*, F. Glorius*, S. Chen*, M. K. Brown*,
Photochemical Dearomative Cycloadditions of Quinolines and Alkenes: Scope and Mechanism Studies,
J. Am. Chem. Soc. 2022, 144, 17680-17691.
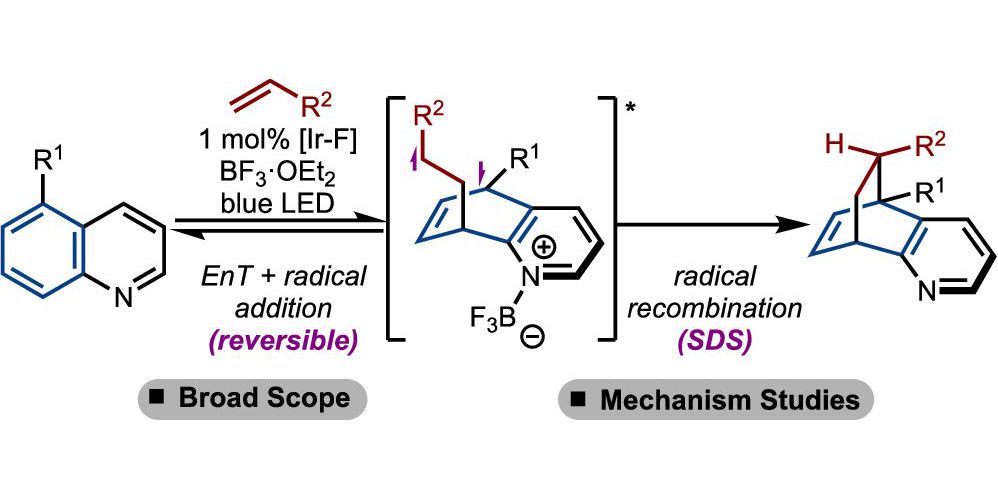
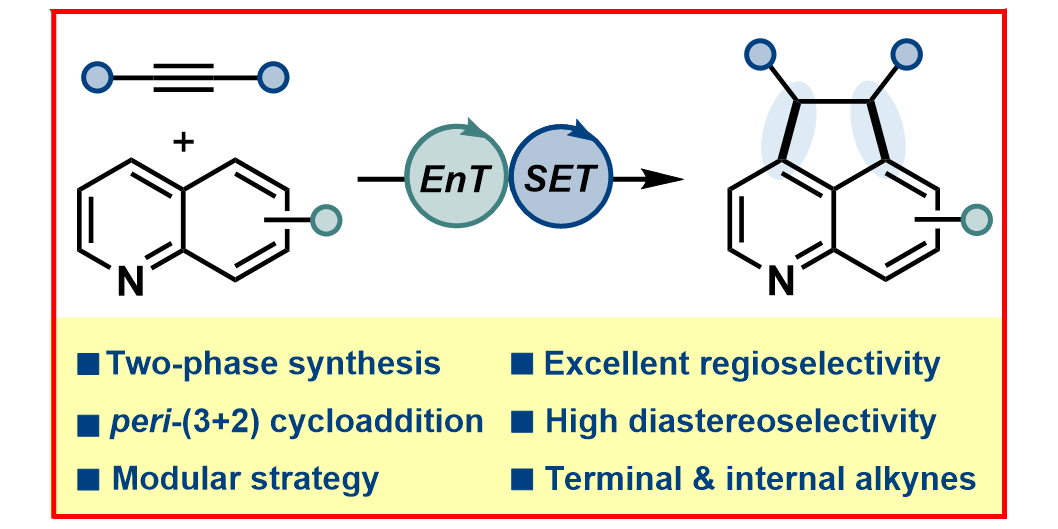
P. Bellotti,§ T. Rogge,§ F. Paulus,§ R. Laskar, N. Rendel, J. Ma, K. N. Houk*, F. Glorius*,
Visible-Light Photocatalyzed peri-(3 + 2) Cycloadditions of Quinolines,
J. Am. Chem. Soc. 2022, 144, 15662-15671.
§ These authors contributed equally
G. Tan, M. Das, H. Keum, P. Bellotti, C. Daniliuc, F. Glorius,
Photochemical single-step synthesis of β-amino acid derivatives from alkenes and (hetero)arenes,
Nature Chem. 2022, 14, 1174-1184.
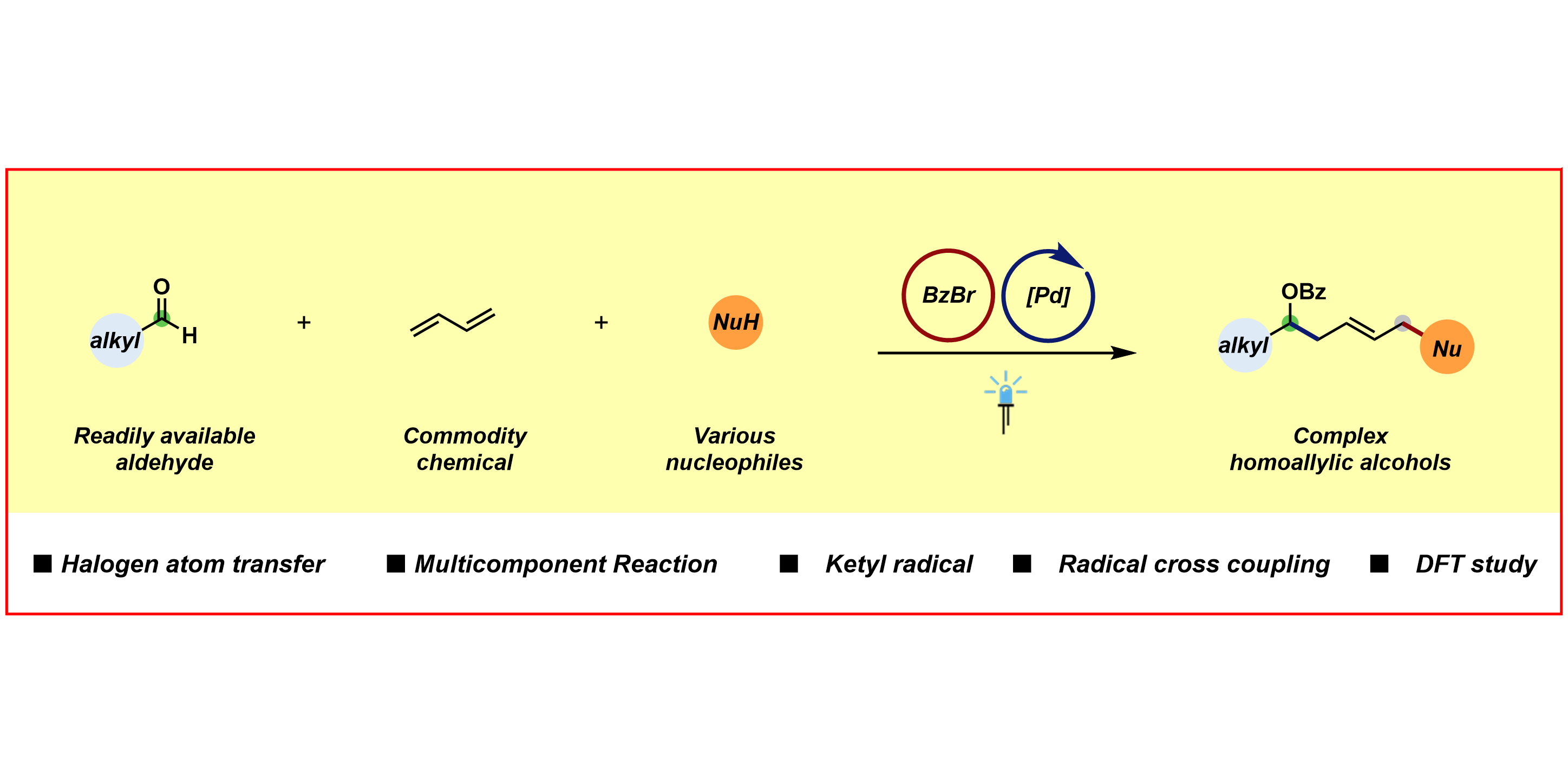
H.-M. Huang,§* P. Bellotti,§ S. Kim, X. Zhang, F. Glorius,*
Catalytic multicomponent reaction involving a ketyl-type radical,
Nature Synth. 2022, 1, 464-474.
§ These authors contributed equally
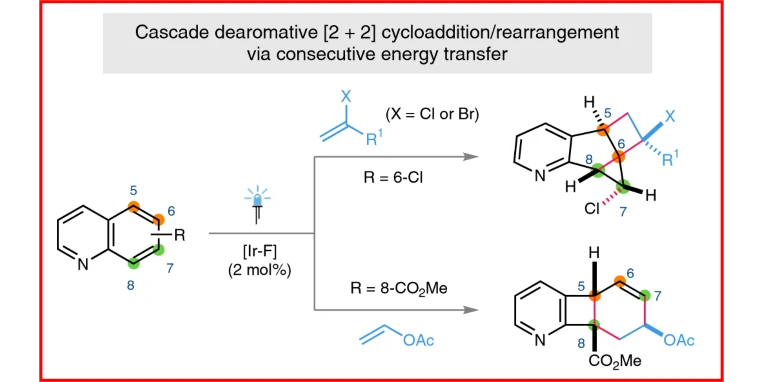
J. Ma,§ S. Chen,§ P. Bellotti,§ T. Wagener, C. Daniliuc, K. N. Houk,* F. Glorius,*
Facile access to fused 2D/3D rings via intermolecular cascade dearomative [2+2] cycloaddition/rearrangement reactions of quinolines with alkenes,
Nature Catal. 2022, 5, 405-413.
§ These authors contributed equally
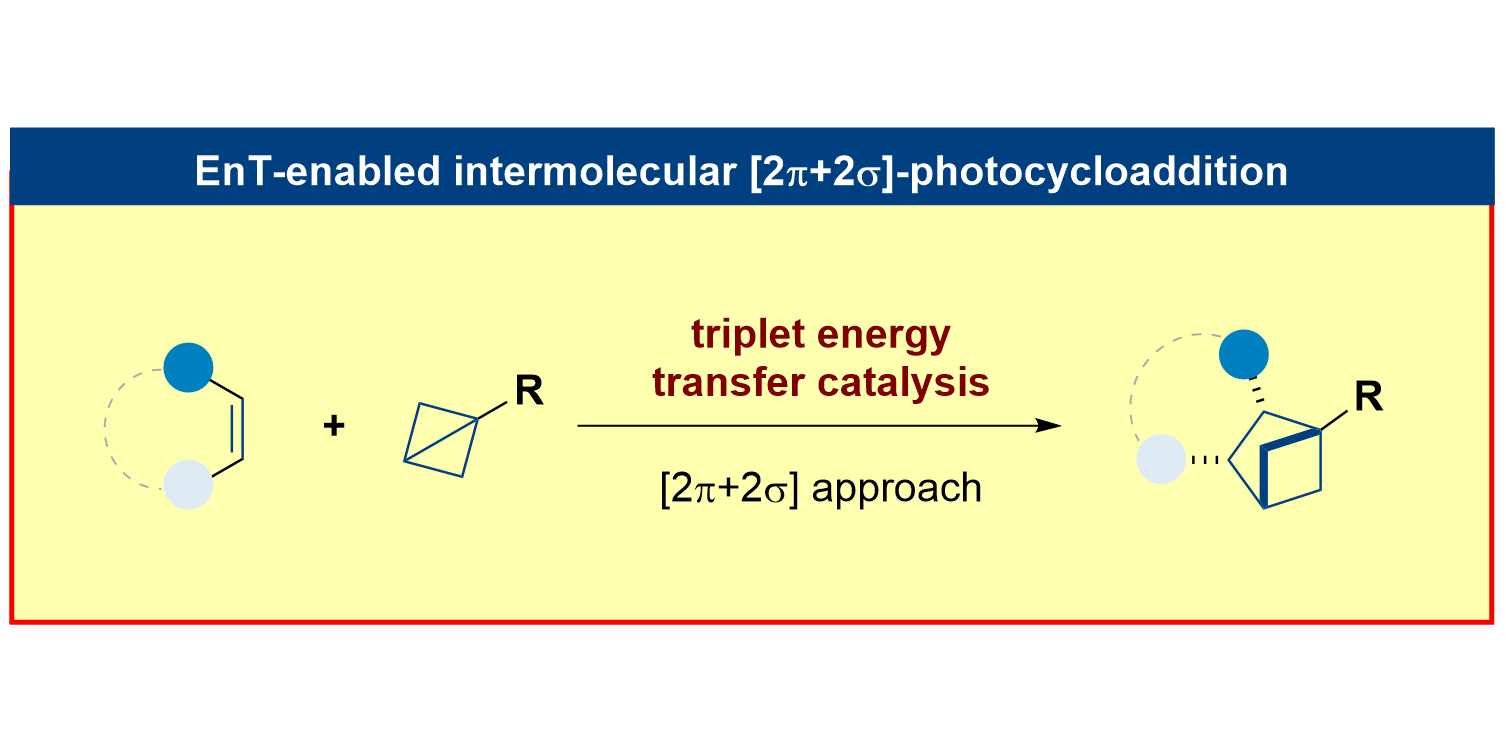
R. Kleinmans,§ T. Pinkert,§ S. Dutta, T. O. Paulisch, H. Keum, C. G. Daniliuc, F. Glorius,
Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer,
Nature 2022, 605, 477-482.
§ These authors contributed equally
L. Quach, S. Dutta, P. M. Pflüger, F. Sandfort, P. Bellotti, F. Glorius,
Visible-Light-Initiated Hydrooxygenation of Unactivated Alkenes - A Strategy for Anti-Markovnikov Hydrofunctionalization,
ACS Catal. 2022, 12, 2499-2504.
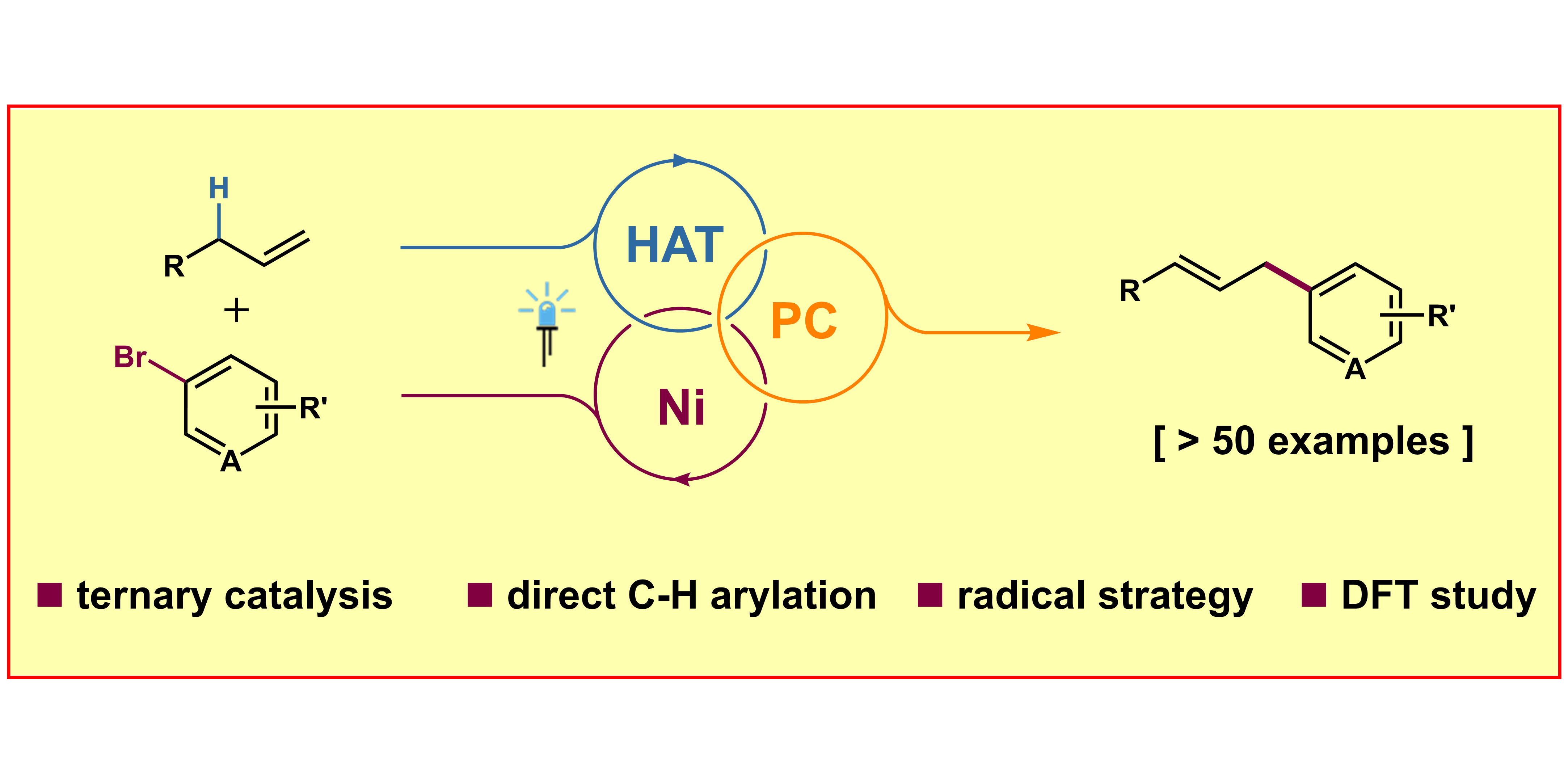
H.-M. Huang,§ P. Bellotti,§ P.-P. Chen,§ K. N. Houk,* F. Glorius,*
Allylic C(sp3)―H Arylation of Olefins via Ternary Catalysis,
Nature Synth. 2022, 1, 59-68.
§ These authors contributed equally
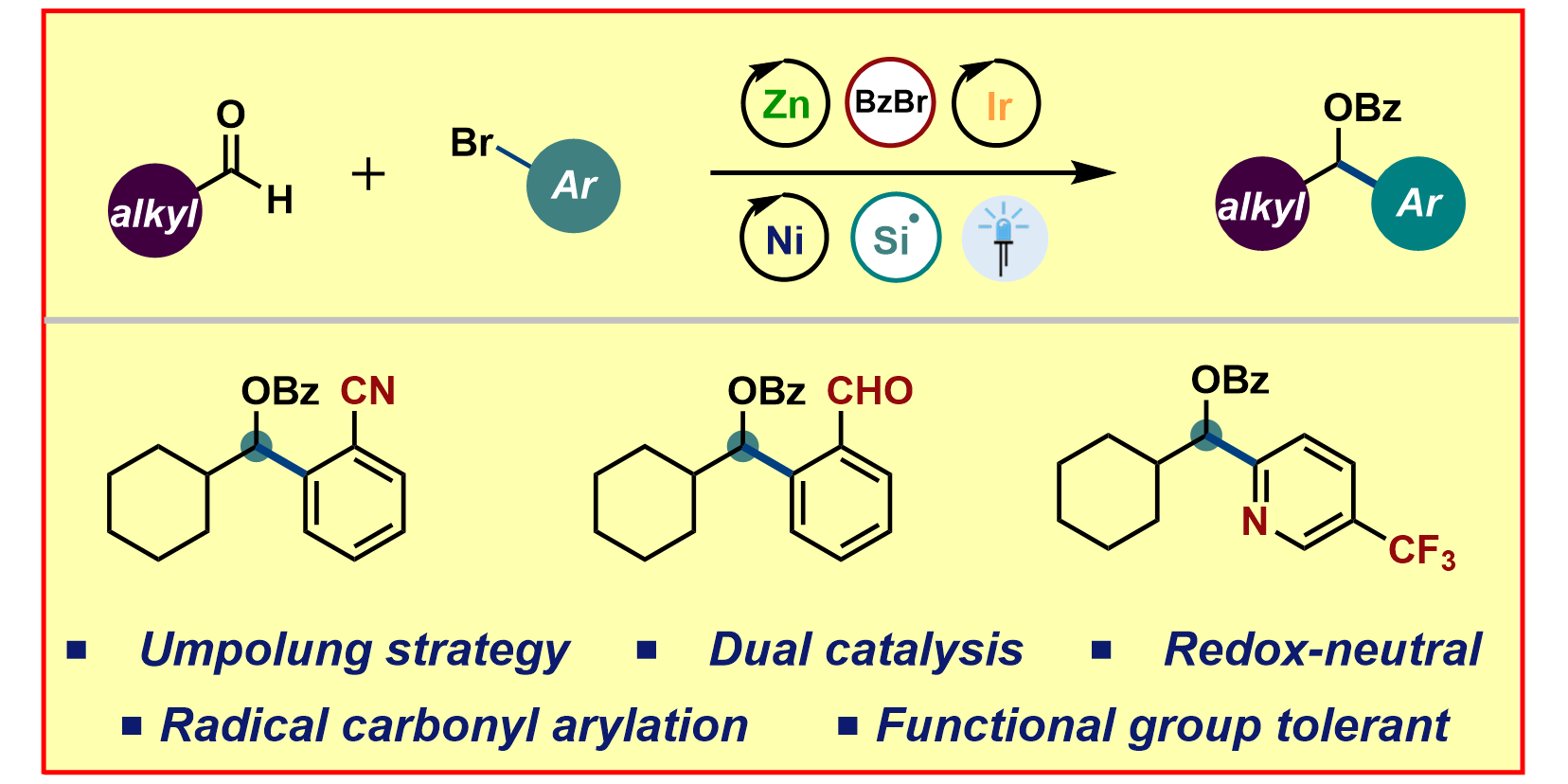
H.-M. Huang,§ P. Bellotti,§ J. E. Erchinger, T. O. Paulisch, F. Glorius,
Radical Carbonyl Umpolung Arylation via Dual Nickel Catalysis,
J. Am. Chem. Soc. 2022, 144,1899-1909.
§ These authors contributed equally
J.-H. Ye, P. Bellotti, C. Heusel, F. Glorius,
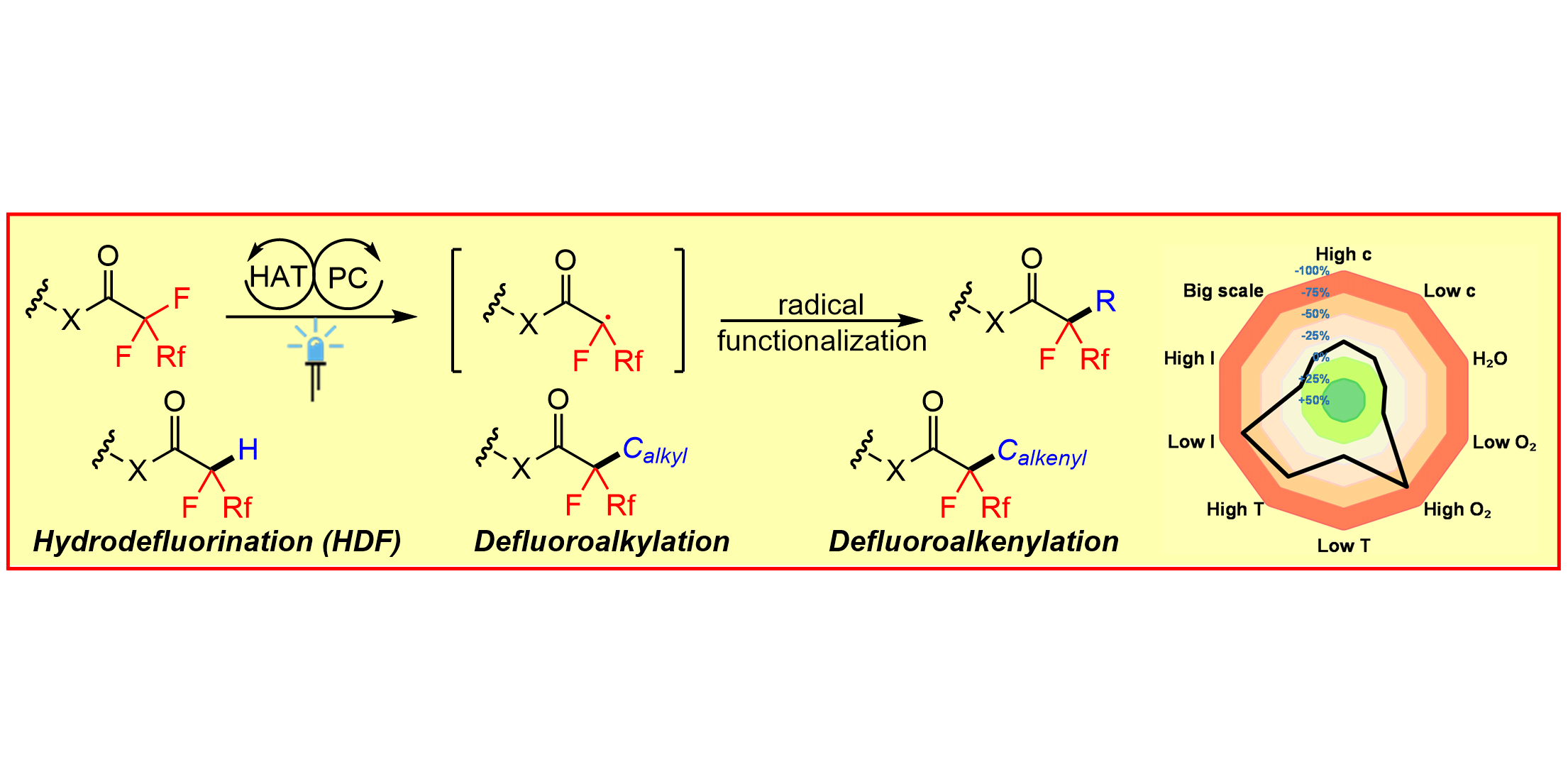
Photoredox-Catalyzed Defluorinative Functionalizations of Polyfluorinated Aliphatic Amides and Esters,
Angew. Chem. Int. Ed. 2022, 61, e202115456; Angew. Chem. 2022, 134 (9), e202115456.
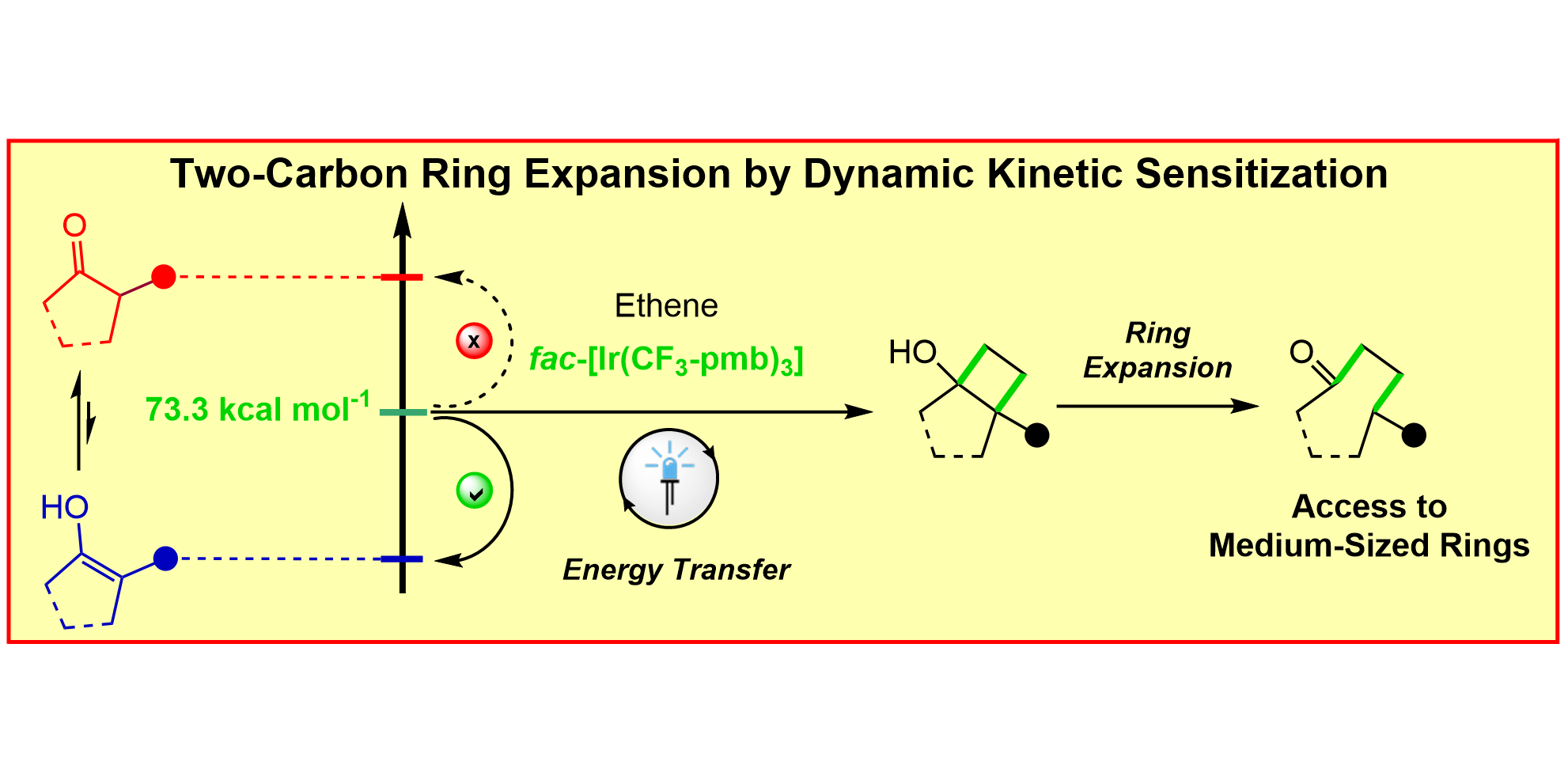
T. O. Paulisch, L. A. Mai, F. Strieth-Kalthoff, M. J. James, C. Henkel, D. M. Guldi,* F. Glorius,*
Dynamic kinetic sensitization of β-dicarbonyl compounds – Access to medium-sized rings via a De Mayo-type ring expansion,
Angew. Chem. Int. Ed. 2022, 61, e202112695; Angew. Chem. 2022, 134, e202112695.
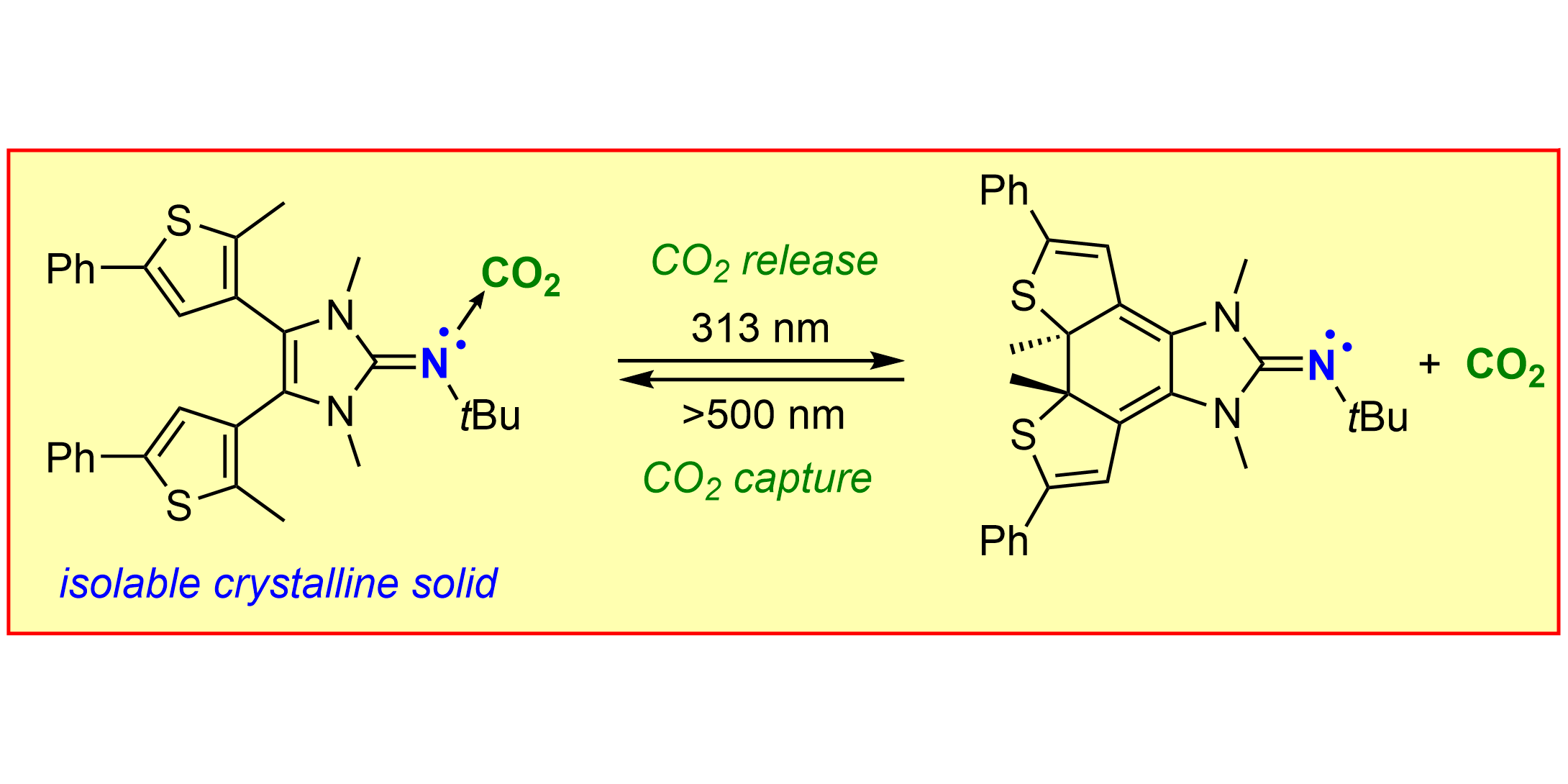
L. F. B. Wilm, M. Das, D. Janssen-Müller, C. Mück-Lichtenfeld, F. Glorius,* F. Dielmann,*
Photoswitchable Nitrogen Superbases: Using Light for Reversible Carbon Dioxide Capture,
Angew. Chem. Int. Ed. 2022, 61, e202112344; Angew. Chem. 2022, 134, e202112344.
Y. Liang,§ F. Strieth-Kalthoff,§ P. Bellotti, F. Glorius,
Catalytic one-carbon homologation of α-amino acids to α-amino aldehydes,
Chem Catal. 2021, 1, 1427-1436.
§ These authors contributed equally
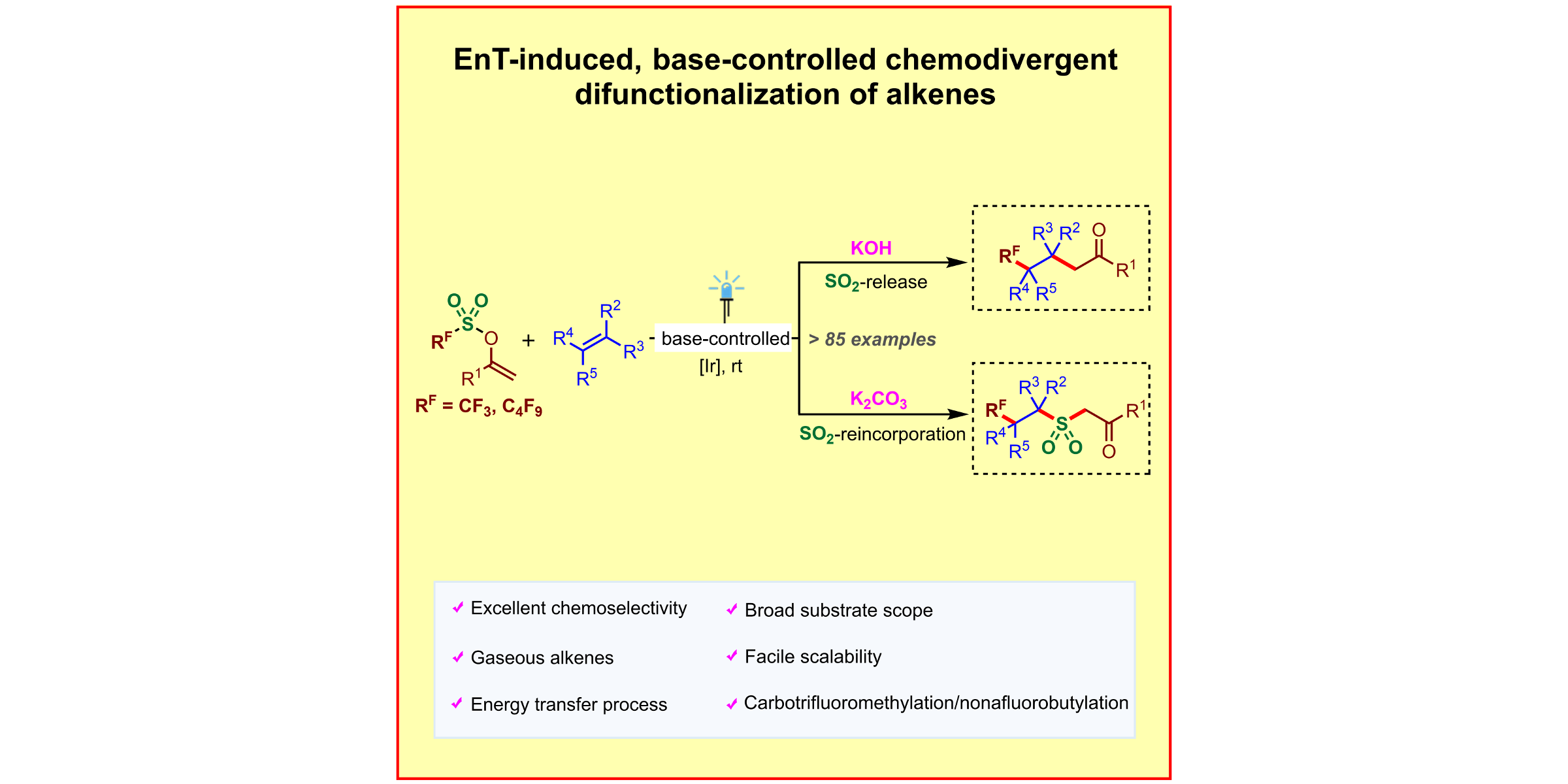
H. Wang, P. Bellotti, X. Zhang, T. O. Paulisch, F. Glorius,
A base-controlled switch of SO2 reincorporation in photocatalyzed radical difunctionalization of alkenes,
Chem 2021, 7, 3412-3424.
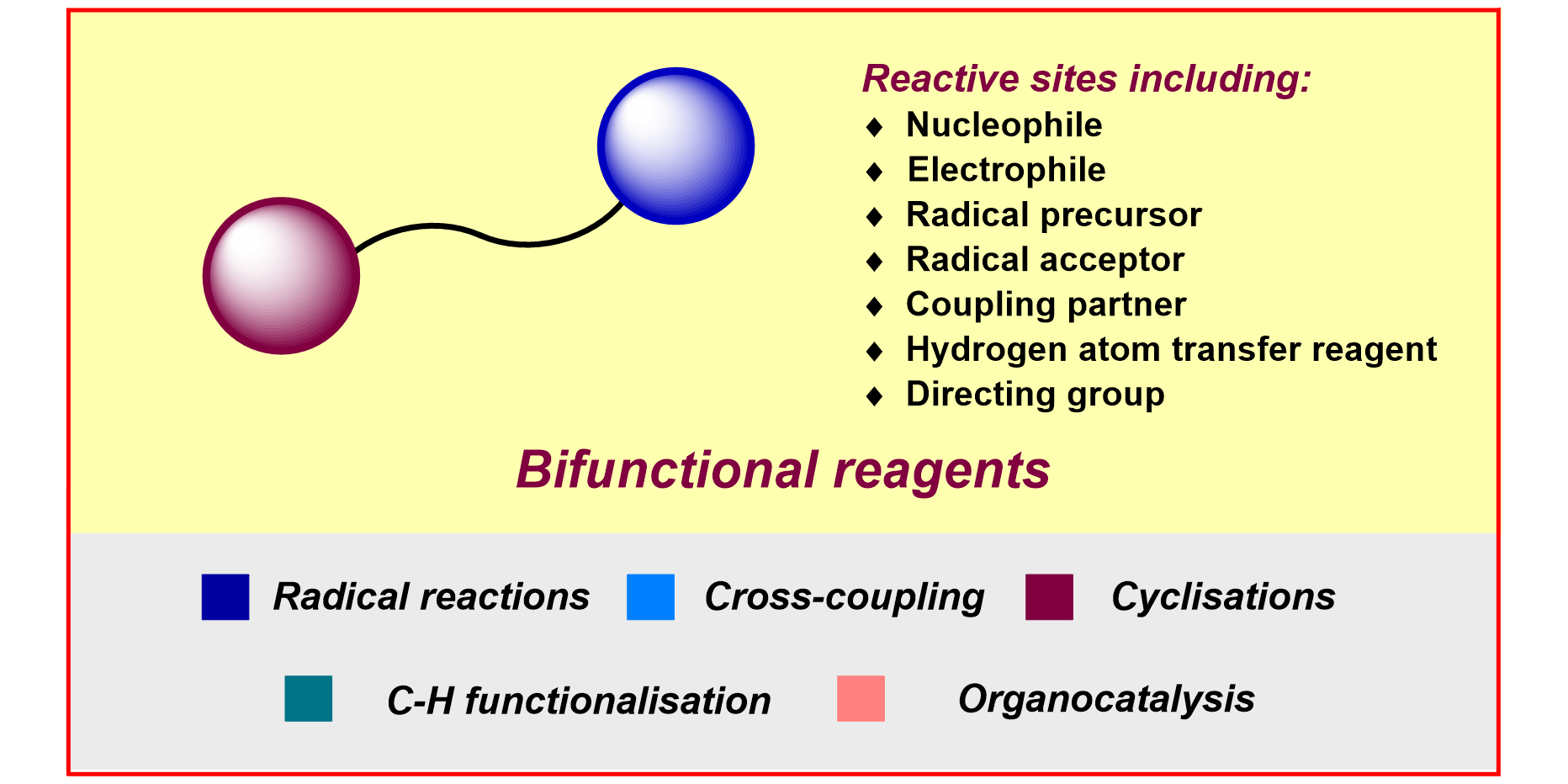
H.-M. Huang,§ P. Bellotti,§ J. Ma, T. Dalton, F. Glorius,
Bifunctional Reagents in Organic Synthesis,
Nature Rev. Chem. 2021, 5, 301-321.
§ These authors contributed equally.
J. Ma,§ S. Chen,§ P. Bellotti,§ R. Guo, F. Schäfer, A. Heusler, X. Zhang, C. Daniliuc, M. K. Brown,* K. N. Houk,* F. Glorius,*
Photochemical Intermolecular Dearomative Cycloaddition of Bicyclic Azaarenes with Alkenes,
Science 2021, 371, 1338-1345.
§ These authors contributed equally.
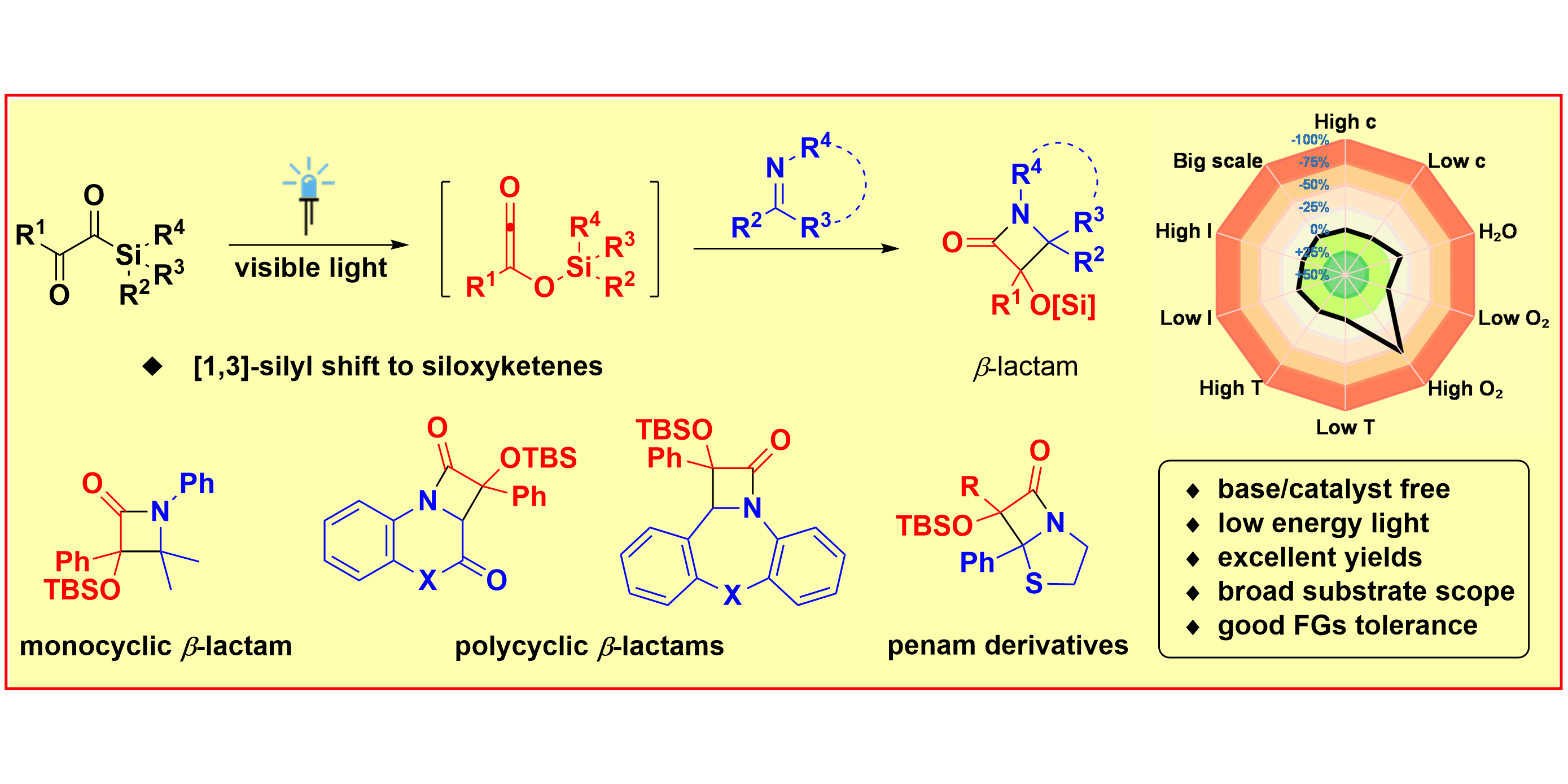
J.-H. Ye, P. Bellotti, T. O. Paulisch, C. G. Daniliuc, F. Glorius,
Visible-Light-Induced Cycloaddition of α-Ketoacylsilanes with Imines: Facile Access to β-Lactams,
Angew. Chem. Int. Ed. 2021, 60, 13671-13676; Angew. Chem. 2021, 133, 13785-13790.
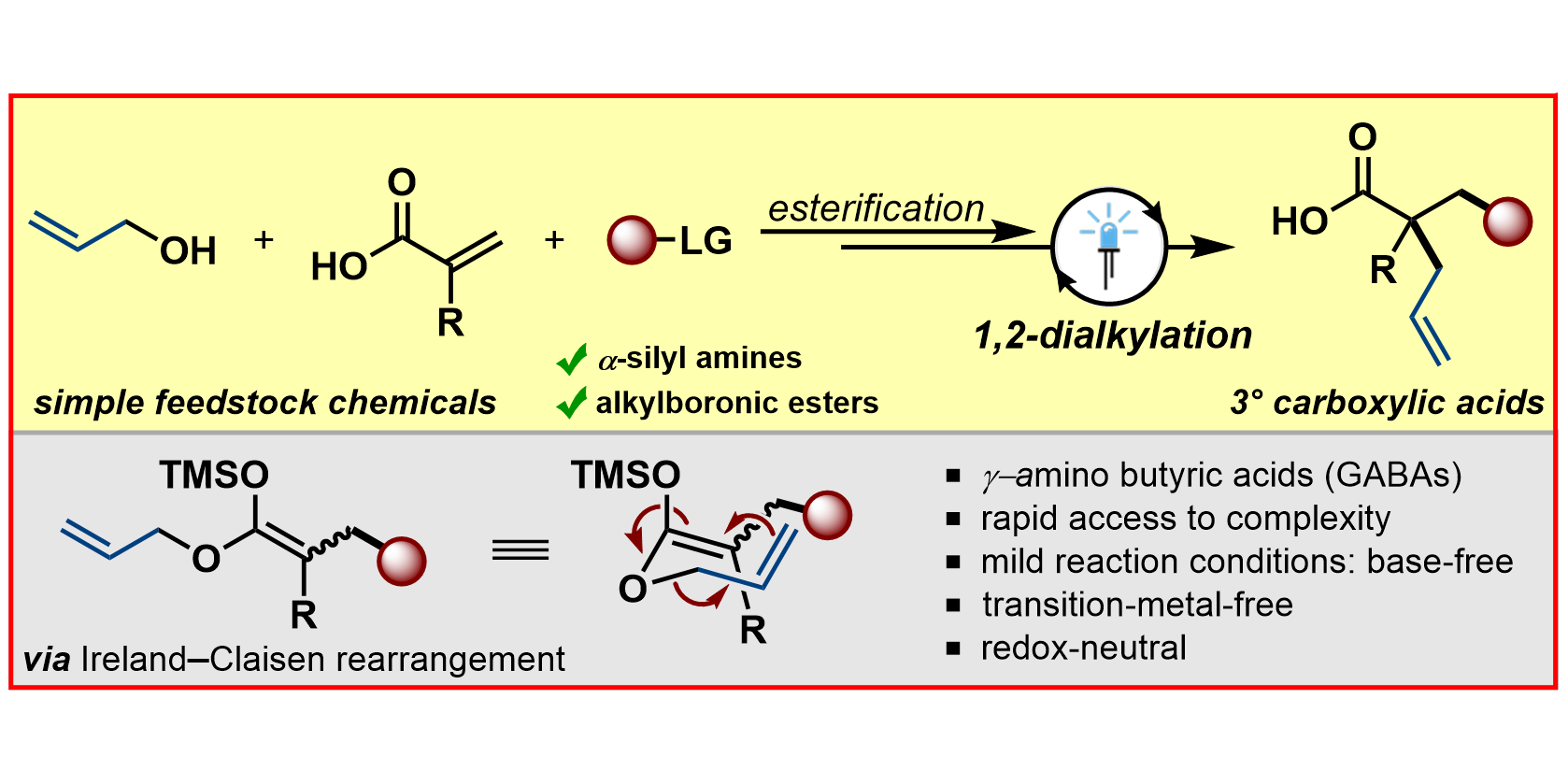
R. Kleinmans, L. E. Will, J. L. Schwarz, F. Glorius,
Photoredox-enabled 1,2-dialkylation of α-substituted acrylates via Ireland–Claisen rearrangement,
Chem. Sci. 2021, 12, 2816-2822.
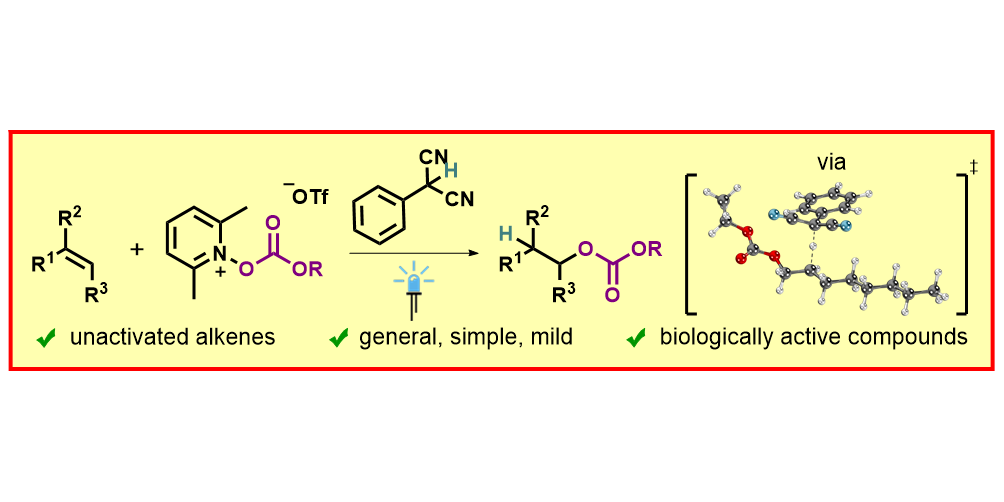
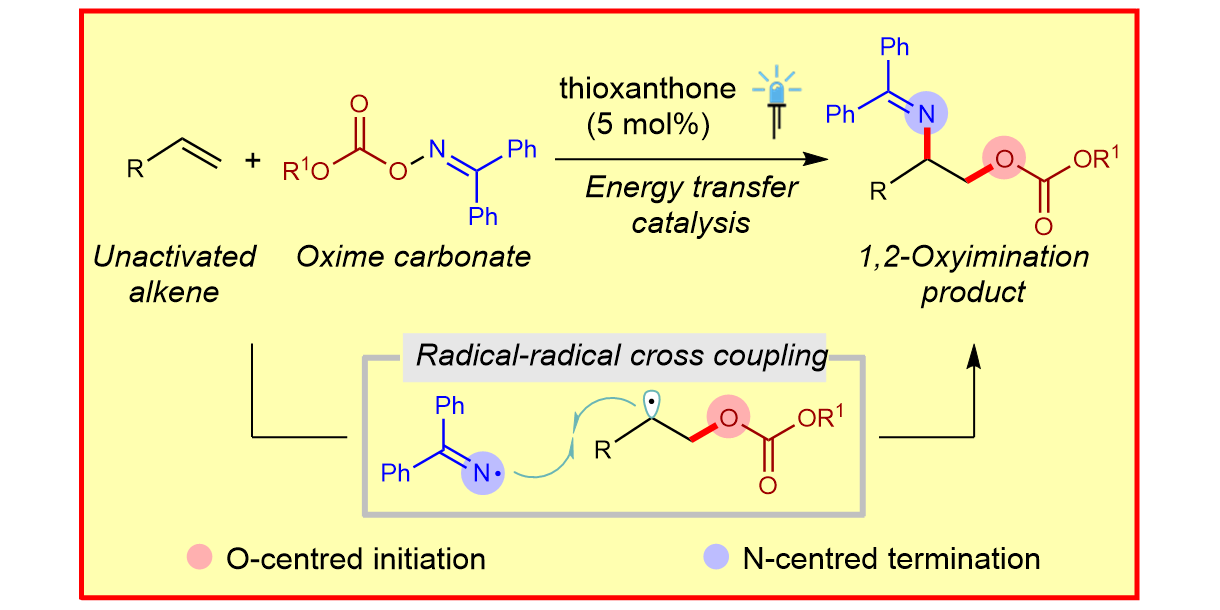
T. Patra, M. Das, C. G. Daniliuc, F. Glorius,
Metal-free, photosensitized oxyimination of unactivated alkenes with bifunctional oxime carbonates,
Nature Catal. 2021, 4, 54-61.
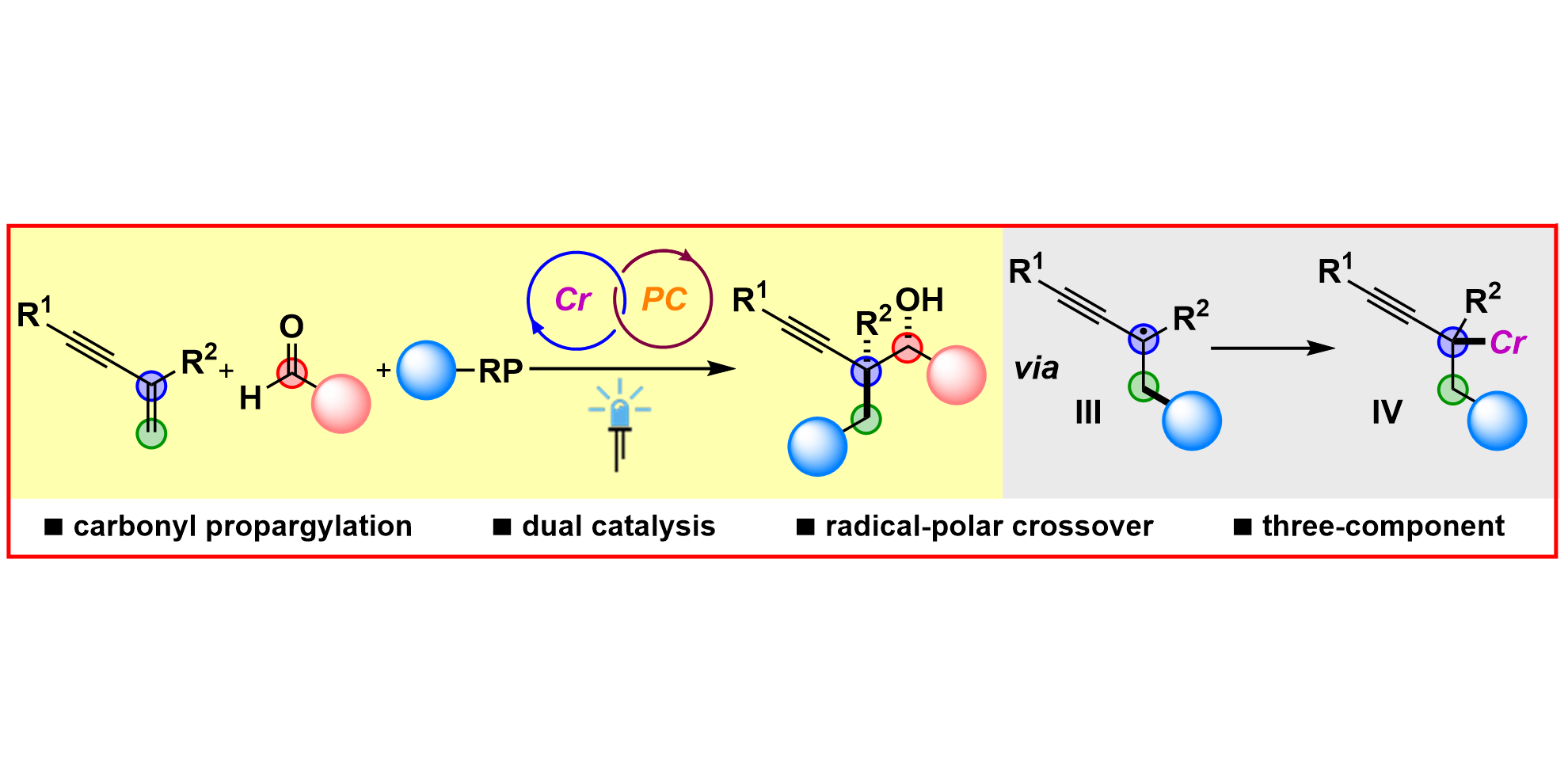
H.-M. Huang, P. Bellotti, C. Daniliuc, F. Glorius,
Radical Carbonyl Propargylation by Dual Catalysis,
Angew. Chem. Int. Ed. 2021, 60, 2464-2471; Angew. Chem. 2021, 133, 2494-2501.
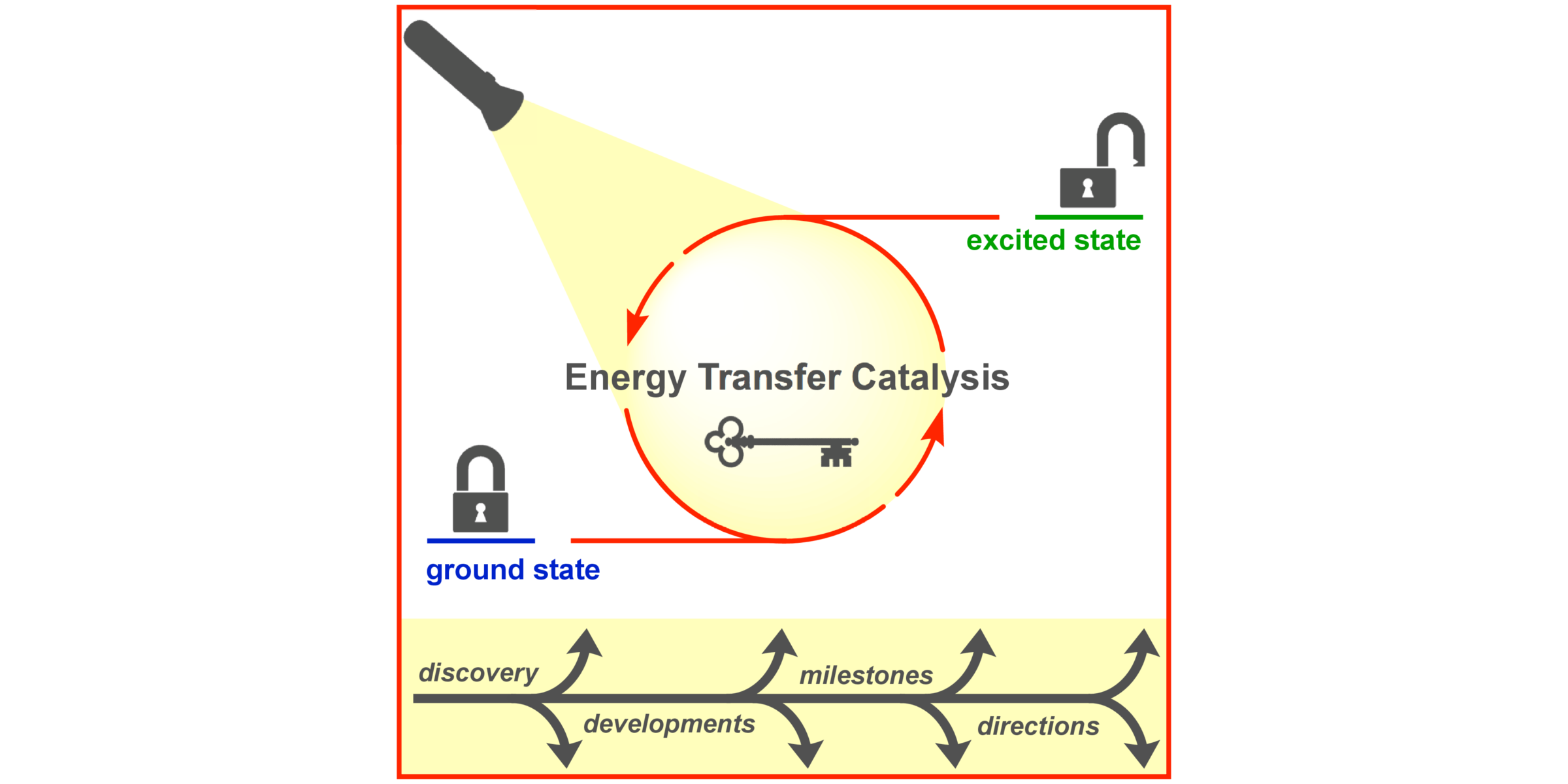
F. Strieth-Kalthoff, F. Glorius,
Triplet Energy Transfer Photocatalysis – Unlocking the Next Level,
Chem 2020, 6, 1888-1903.
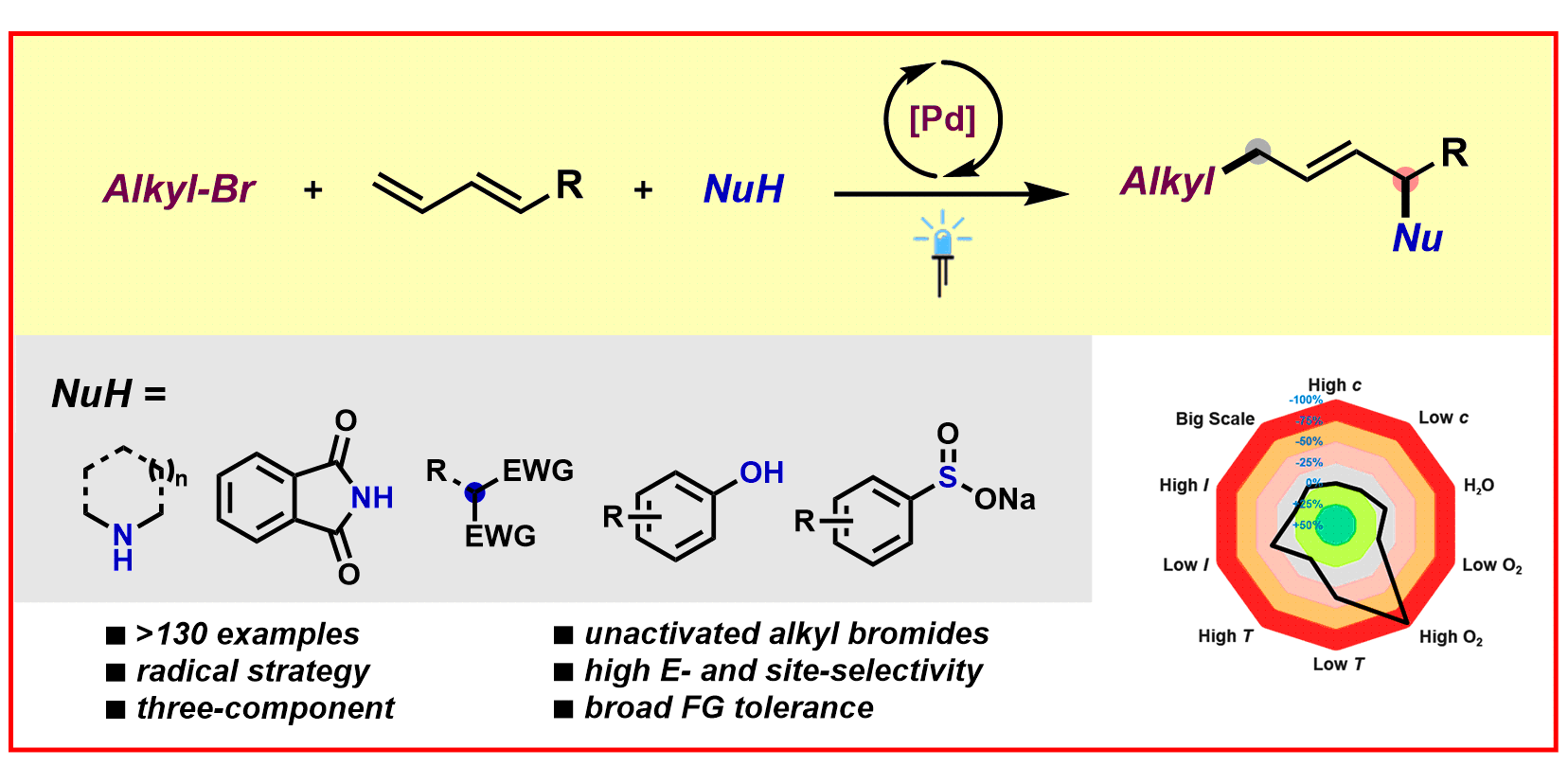
H.-M. Huang, P. Bellotti, P. M. Pflueger, J. L. Schwarz, B. Heidrich, F. Glorius,
A Three-Component, Interrupted Radical Heck/Allylic Substitution Cascade Involving Unactivated Alkyl Bromides,
J. Am. Chem. Soc. 2020, 142, 10173-10183.
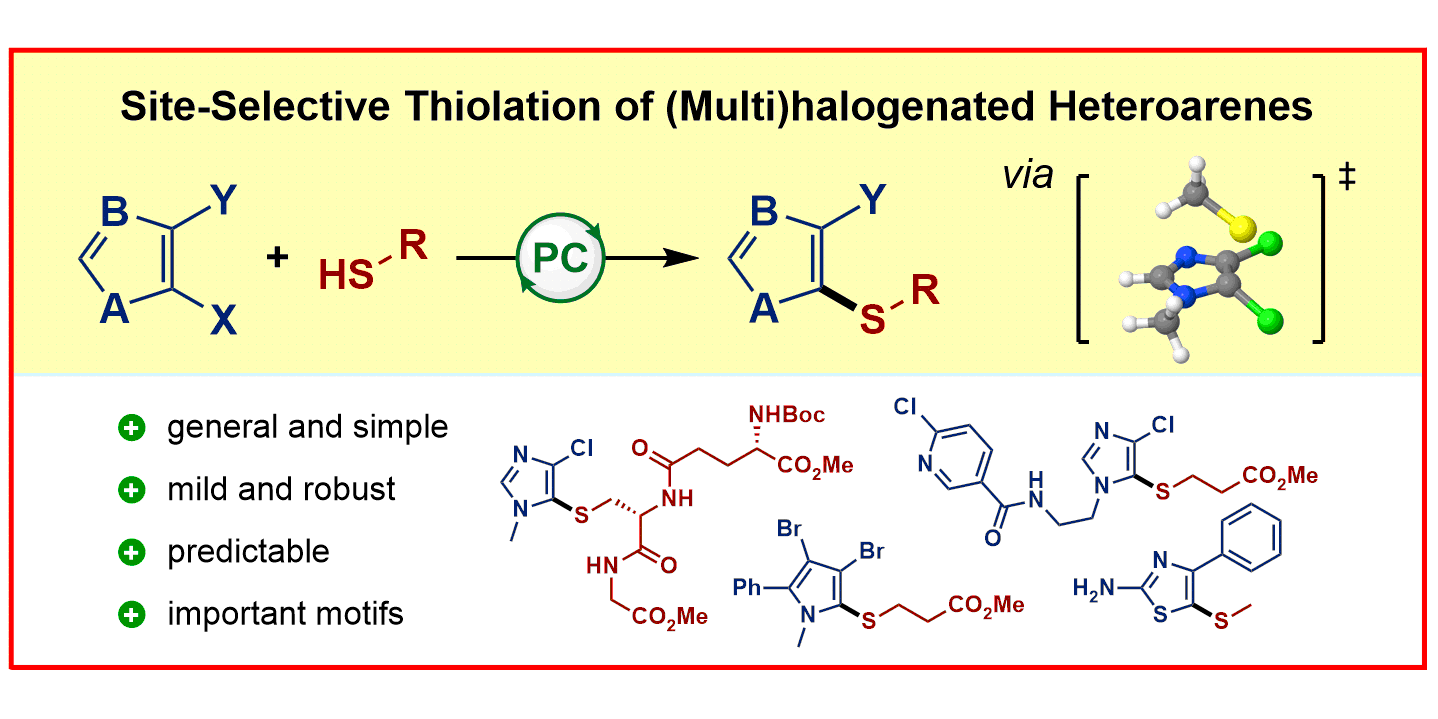
F. Sandfort, T. Knecht, T. Pinkert, C. G. Daniliuc, F. Glorius,
Site-Selective Thiolation of (Multi)halogenated Heteroarenes,
J. Am. Chem. Soc. 2020, 142, 6913-6919.
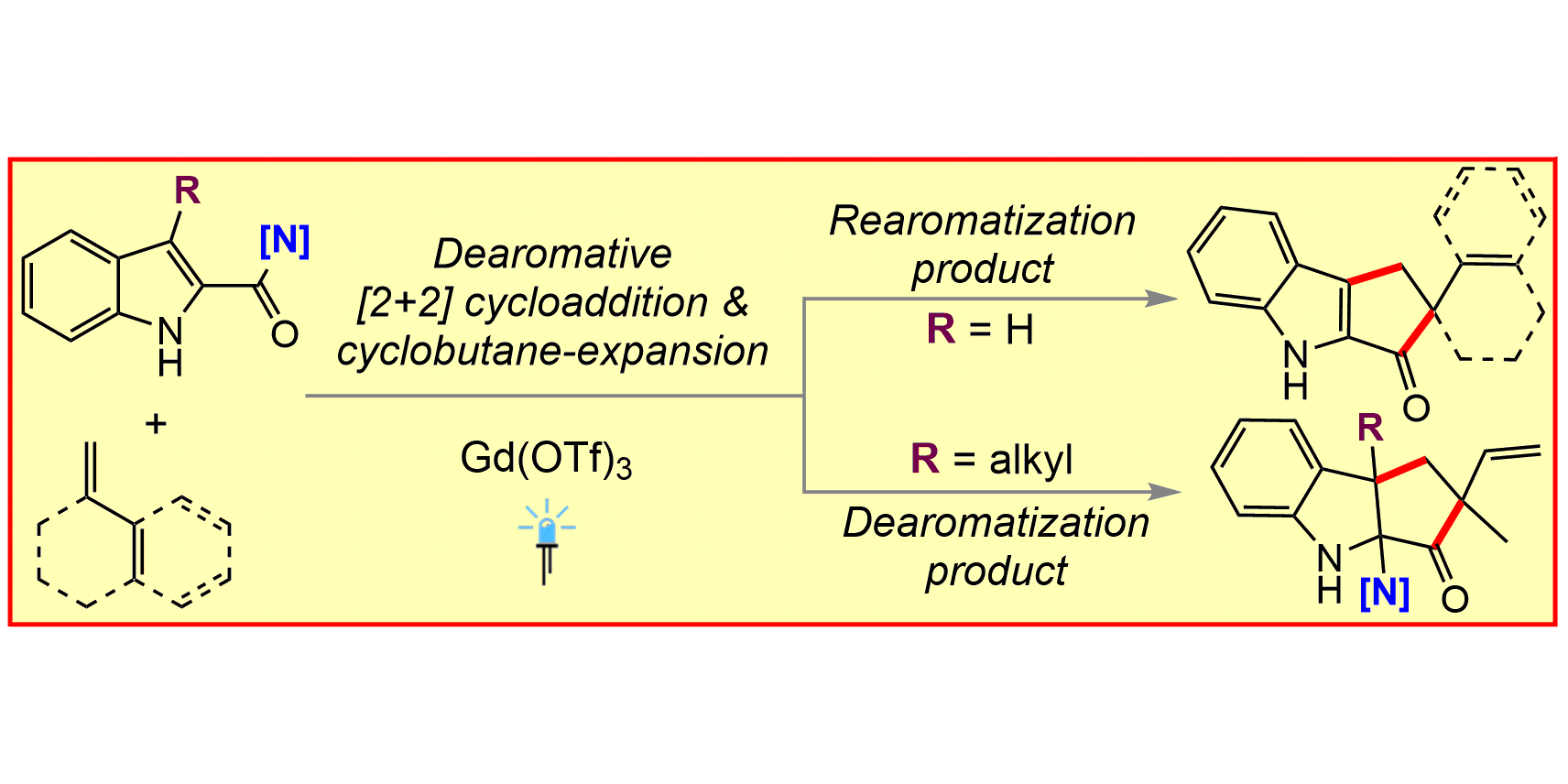
J. Ma, F. Schäfers, C. Daniliuc, K. Bergander, C. A. Strassert, F. Glorius,
Gadolinium Photocatalysis: Dearomative [2+2] Cycloaddition/Ring-Expansion Sequence with Indoles,
Angew. Chem. Int. Ed. 2020, 59, 9639-9645; Angew. Chem. 2020, 132, 9726-9735.
H.-M. Huang , M. Koy, E. Serrano, P. M. Pflüger, J. L. Schwarz, F. Glorius,
Catalytic radical generation of π-allylpalladium complexes,
Nature Catalysis 2020, 3, 393-400.
J. L. Schwarz,§ R. Kleinmans,§ T. O. Paulisch, F. Glorius,
1,2-Amino Alcohols via Cr/Photoredox Dual-Catalyzed Addition of α‑Amino Carbanion Equivalents to Carbonyls,
J. Am. Chem. Soc. 2020, 142, 2168-2174.
§ Both authors contributed equally.
T. Patra, P. Bellotti, F. Glorius,
Photosensitized intermolecular carboimination of alkenes through persistent radical effect,
Angew. Chem. Int. Ed. 2020, 59, 3172-3177; Angew. Chem. 2020, 132, 3198-3203.
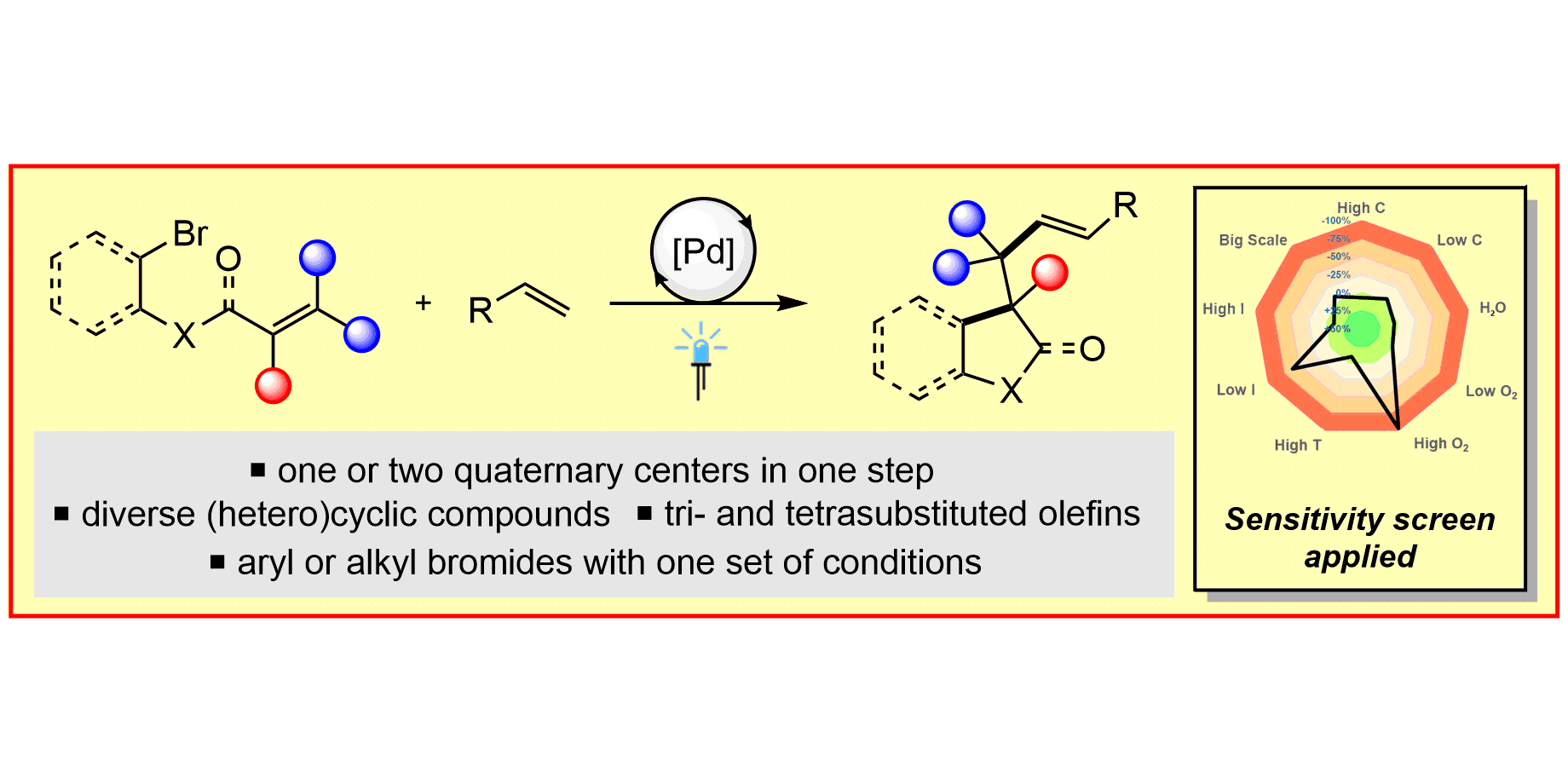
M. Koy,§ P. Bellotti,§ F. Katzenburg, C. Daniliuc, F. Glorius,
Synthesis of All‐Carbon Quaternary Centers by Palladium‐Catalyzed Olefin Dicarbofunctionalization,
Angew. Chem. Int. Ed. 2020, 59, 2375-2379; Angew. Chem. 2020, 132, 2395-2399.
§ Both authors contributed equally.
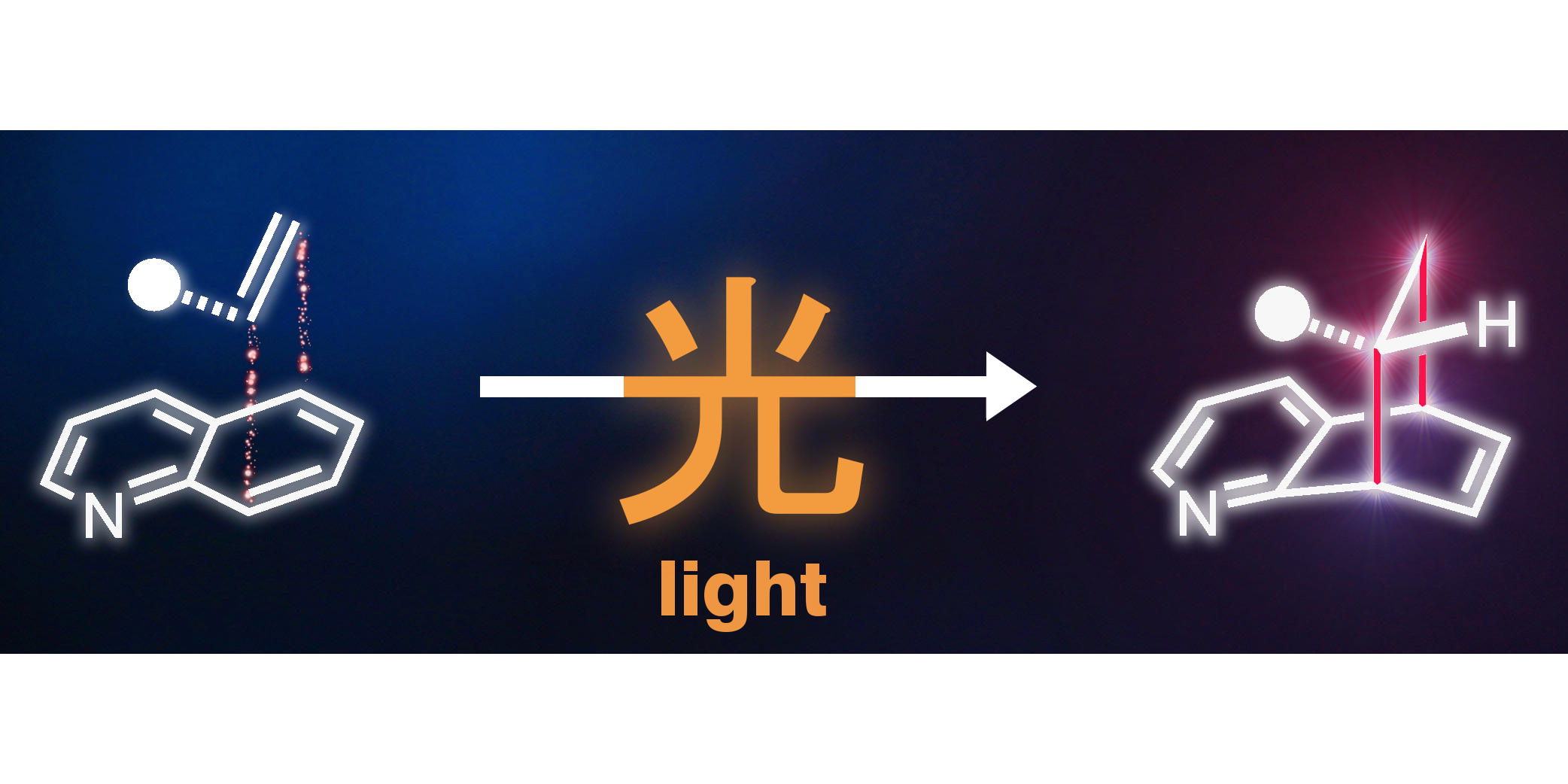
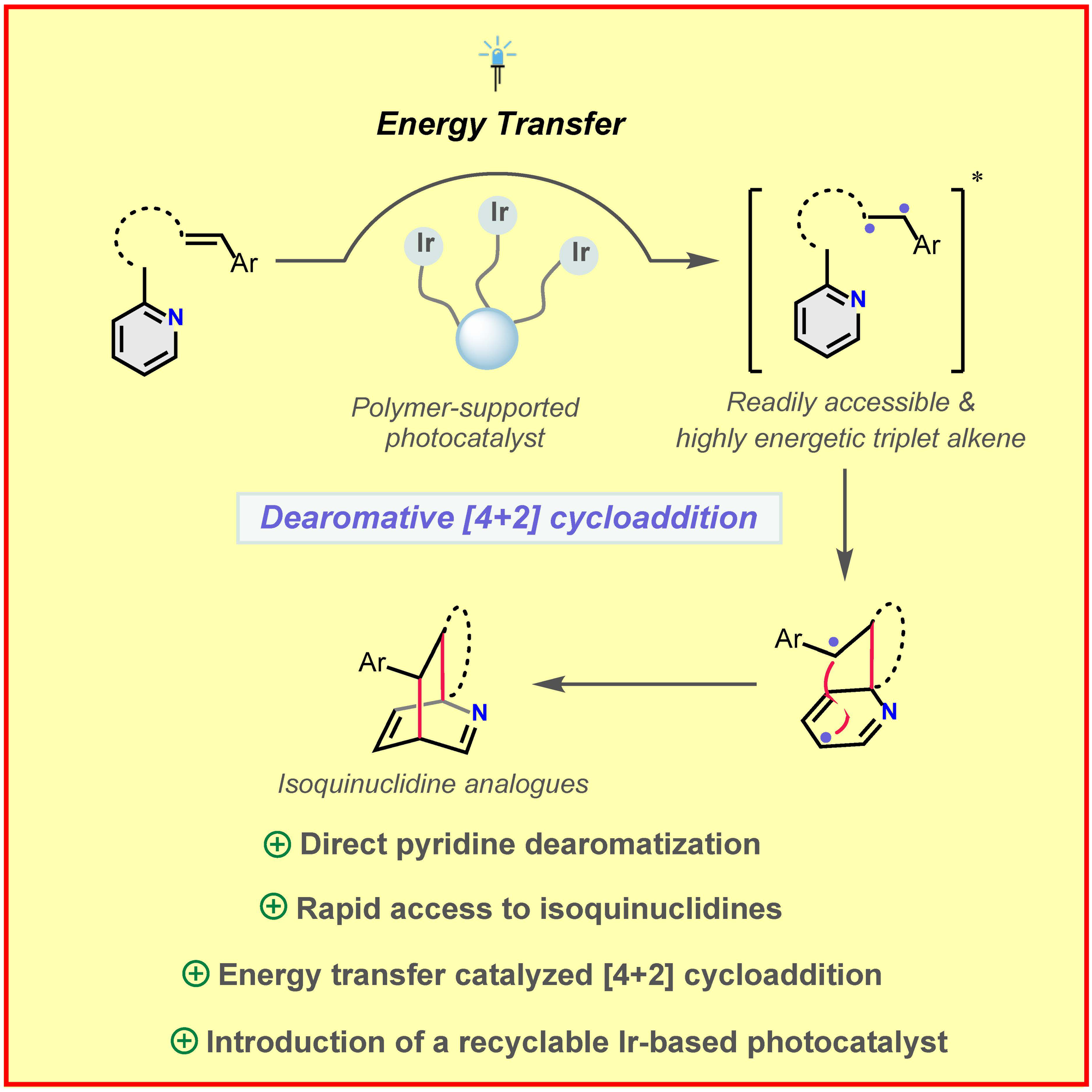
J. Ma, F. Strieth-Kalthoff, T. Dalton, M. Freitag, J. L. Schwarz, K. Bergander, C. Daniliuc, F. Glorius,
Direct Dearomatization of Pyridines via an Energy-Transfer-Catalyzed Intramolecular [4+2] Cycloaddition,
Chem 2019, 5, 2854-2864 (free featured article).
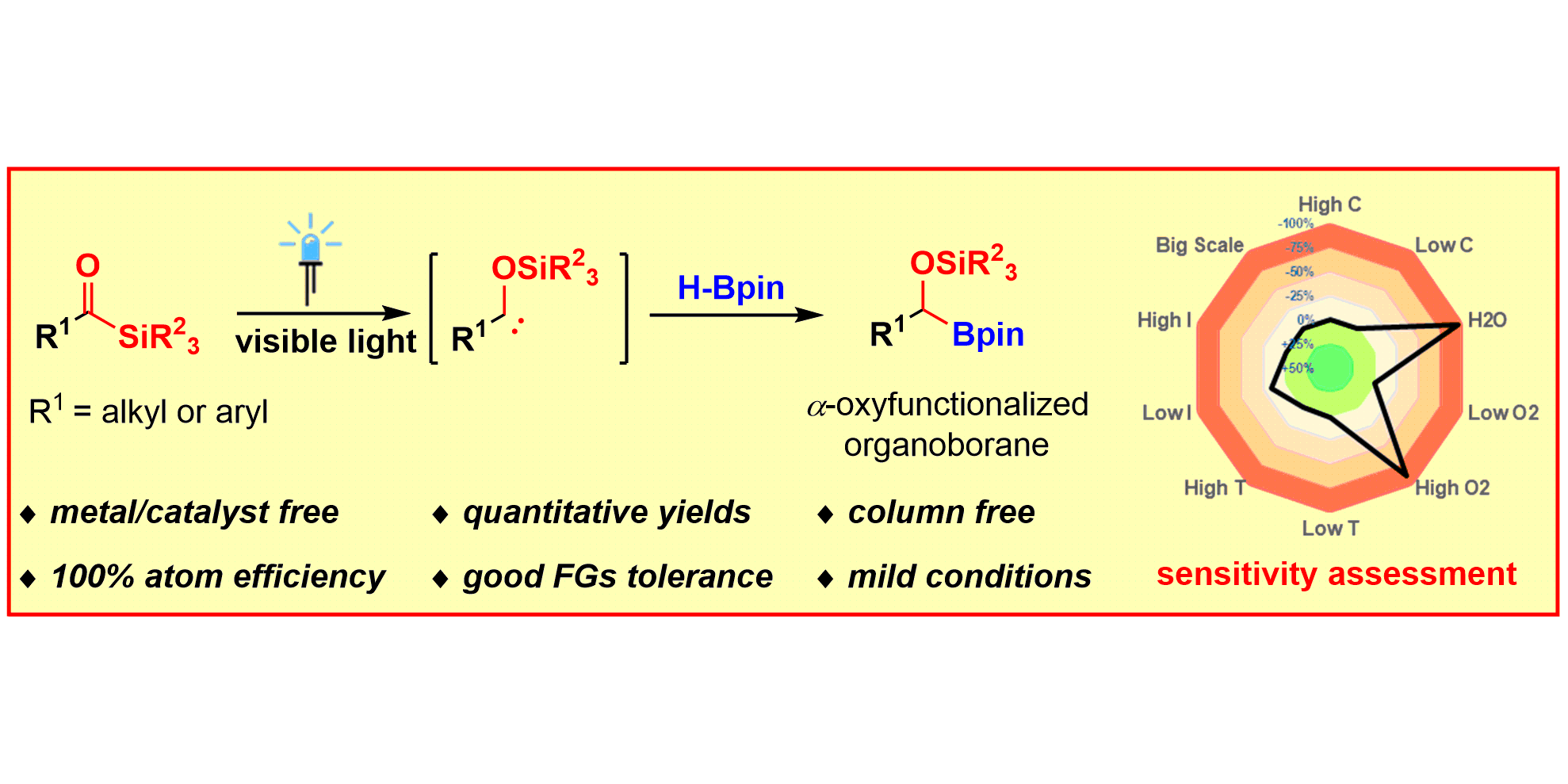
J.-H. Ye, L. Quach, T. Paulisch, F. Glorius,
Visible-Light-Induced, Metal-Free Carbene Insertion into B–H Bonds between Acylsilanes and Pinacolborane,
J. Am. Chem. Soc. 2019, 141, 16227-16231.
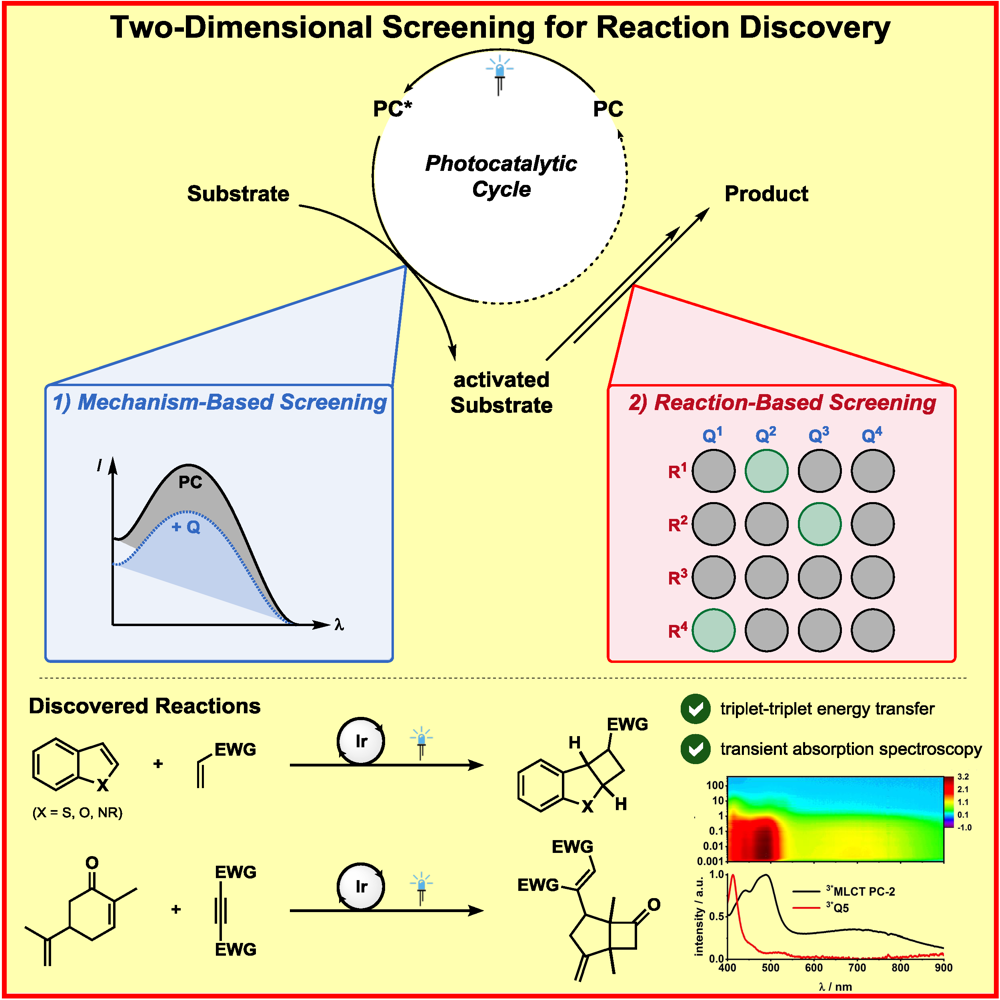
F. Strieth-Kalthoff, C. Henkel, M. Teders, A. Kahnt, W. Knolle, A. Gómez-Suárez, K. Dirian, W. Alex, K. Bergander, C. G. Daniliuc, B. Abel, D. M. Guldi,* F. Glorius,*
Discovery of Unforeseen Energy-Transfer-Based Transformations Using a Combined Screening Approach,
Chem 2019, 5, 2183-2194.
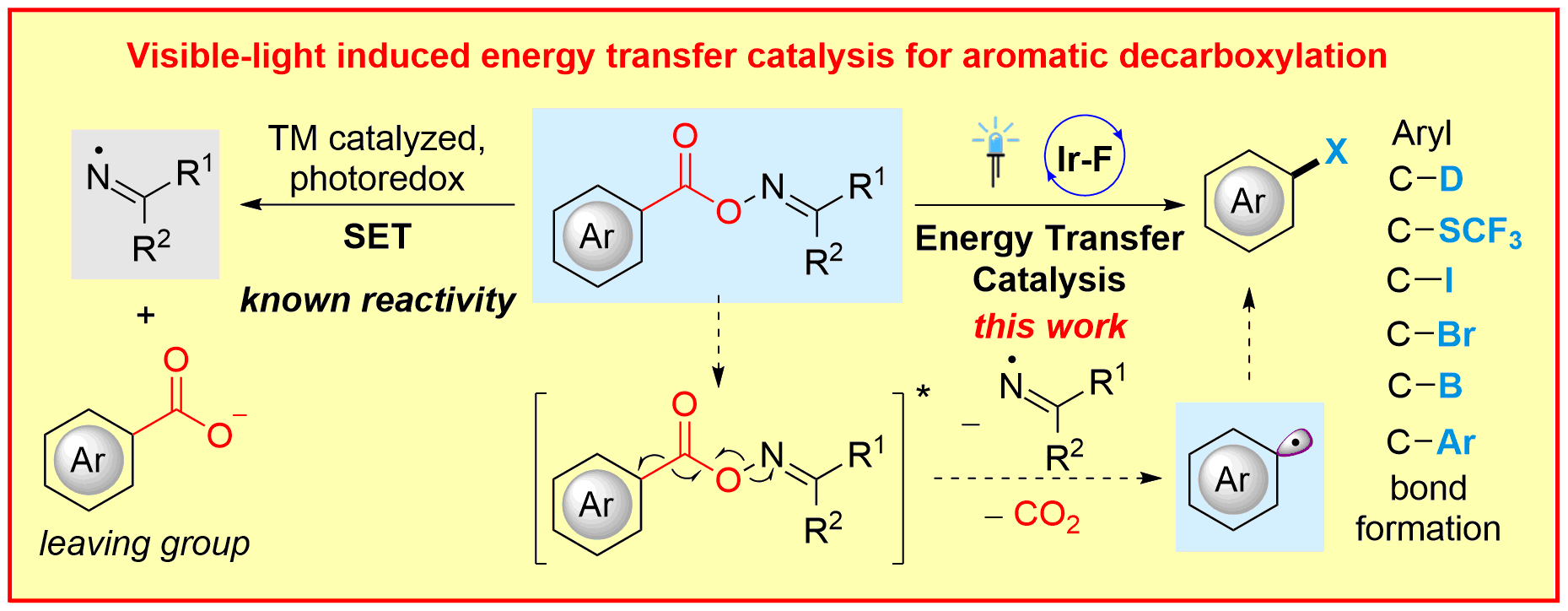
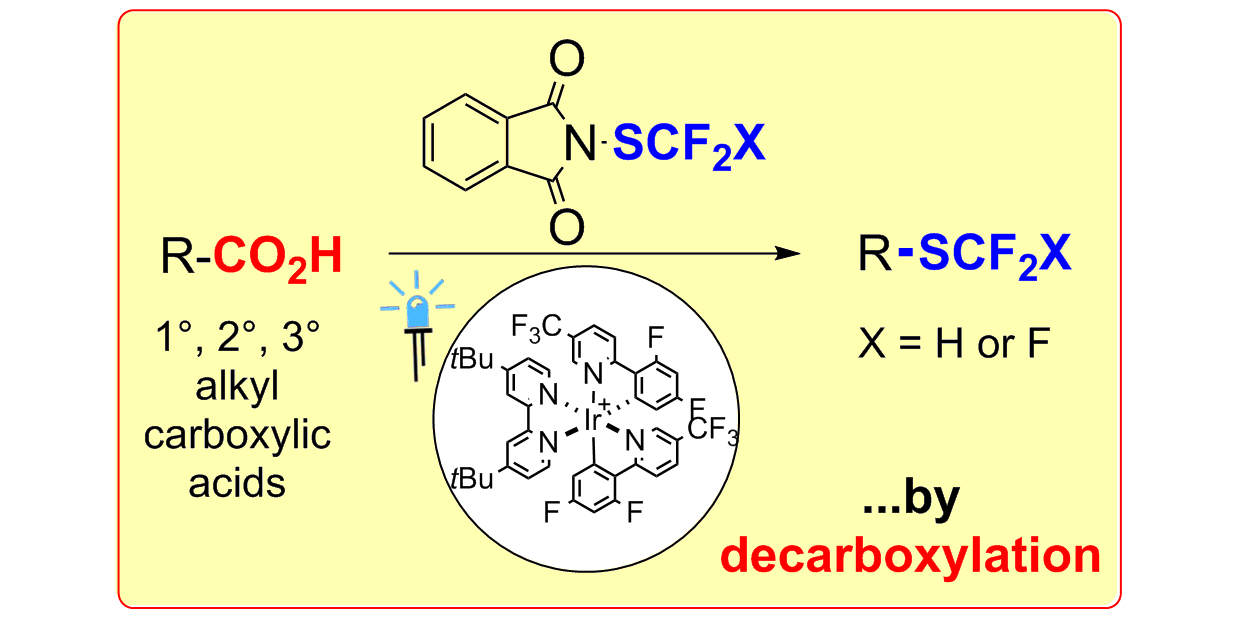
T. Patra, S. Mukherjee, J. Ma, F. Strieth-Kalthoff, F. Glorius,
Visible-light photosensitized aryl and alkyl decarboxylative carbon-heteroatom and carbon-carbon bond formations,
Angew. Chem. Int. Ed. 2019, 58, 10514-10520; Angew. Chem. 2019, 131, 10624-10630.
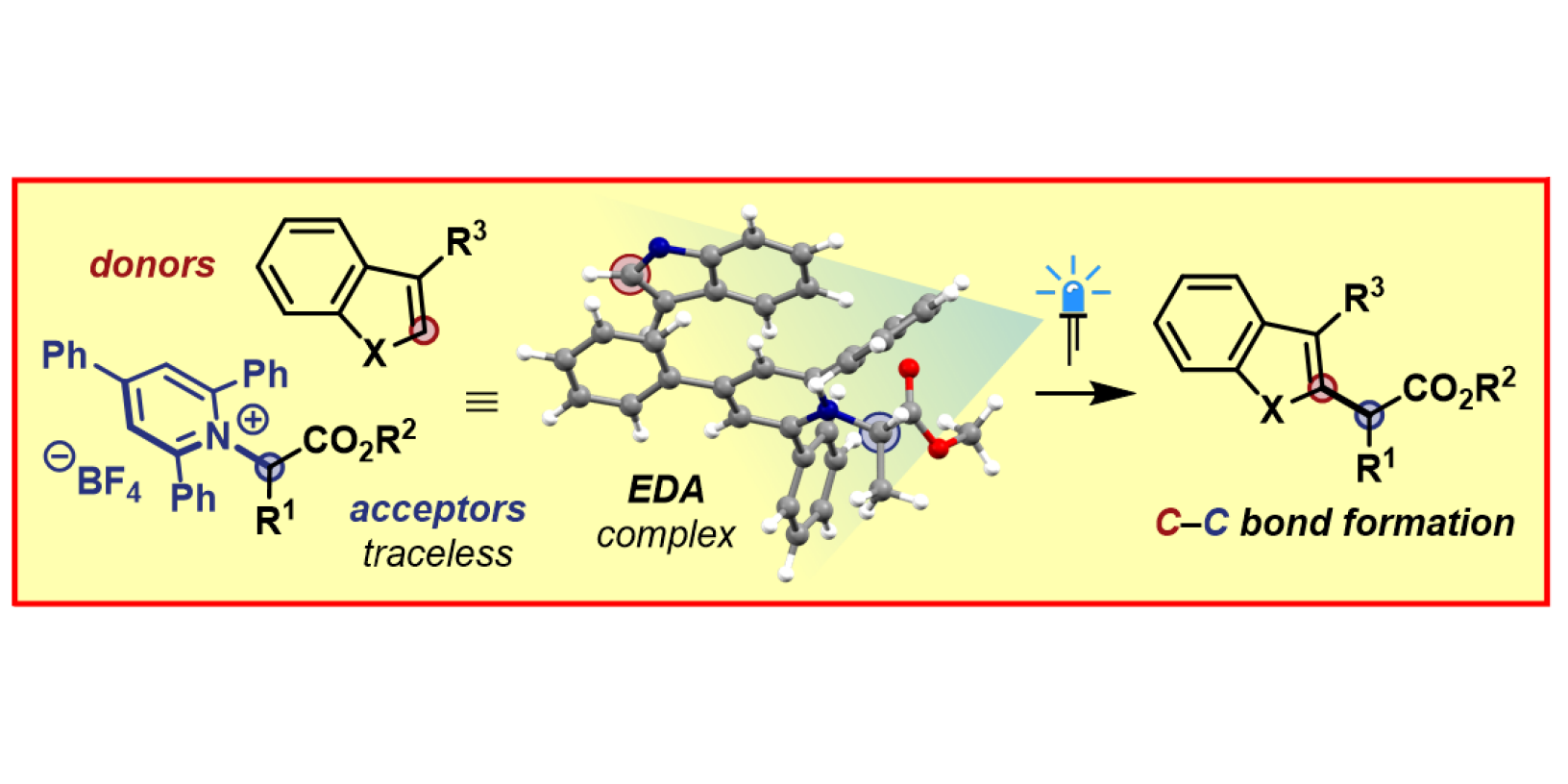
M. J. James, F. Strieth-Kalthoff, F. Sandfort, F. J. R. Klauck, F. Wagener, F. Glorius,
Visible-Light-Mediated Charge Transfer Enables C–C Bond Formation with Traceless Acceptor Groups,
Chem. Eur. J. 2019, 25, 8240-8244.
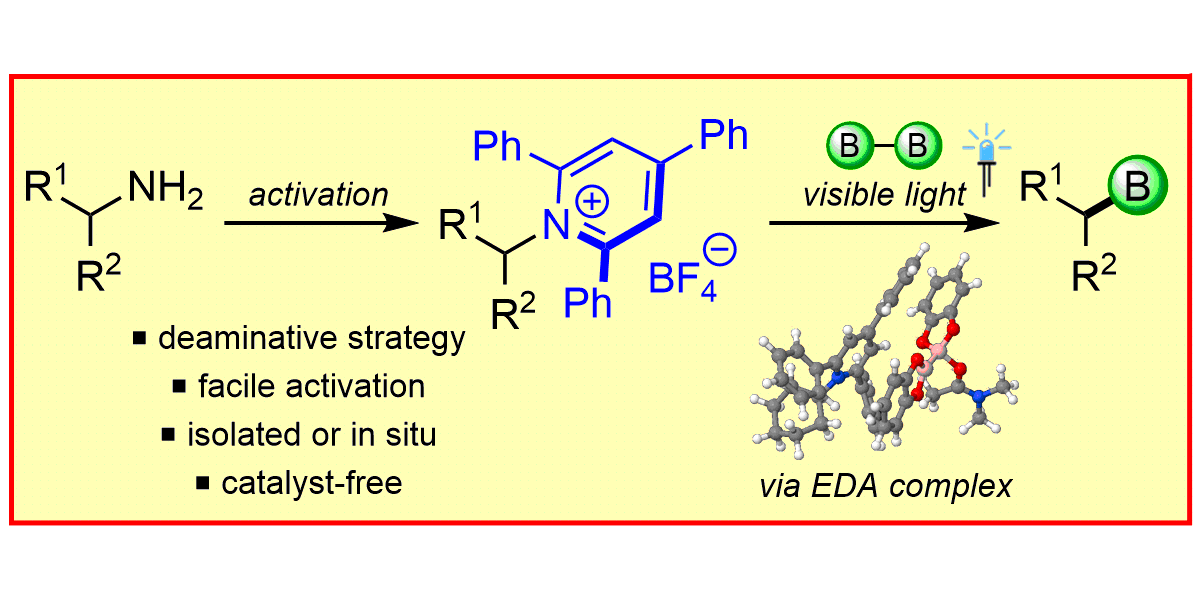
F. Sandfort,§ F. Strieth-Kalthoff,§ F. J. R. Klauck, M. J. James, F. Glorius,
Deaminative Borylation of Aliphatic Amines Enabled by Visible Light Excitation of an Electron-Donor-Acceptor Complex,
Chem. Eur. J. 2018, 24, 17210-17214.
§ Both authors contributed equally.
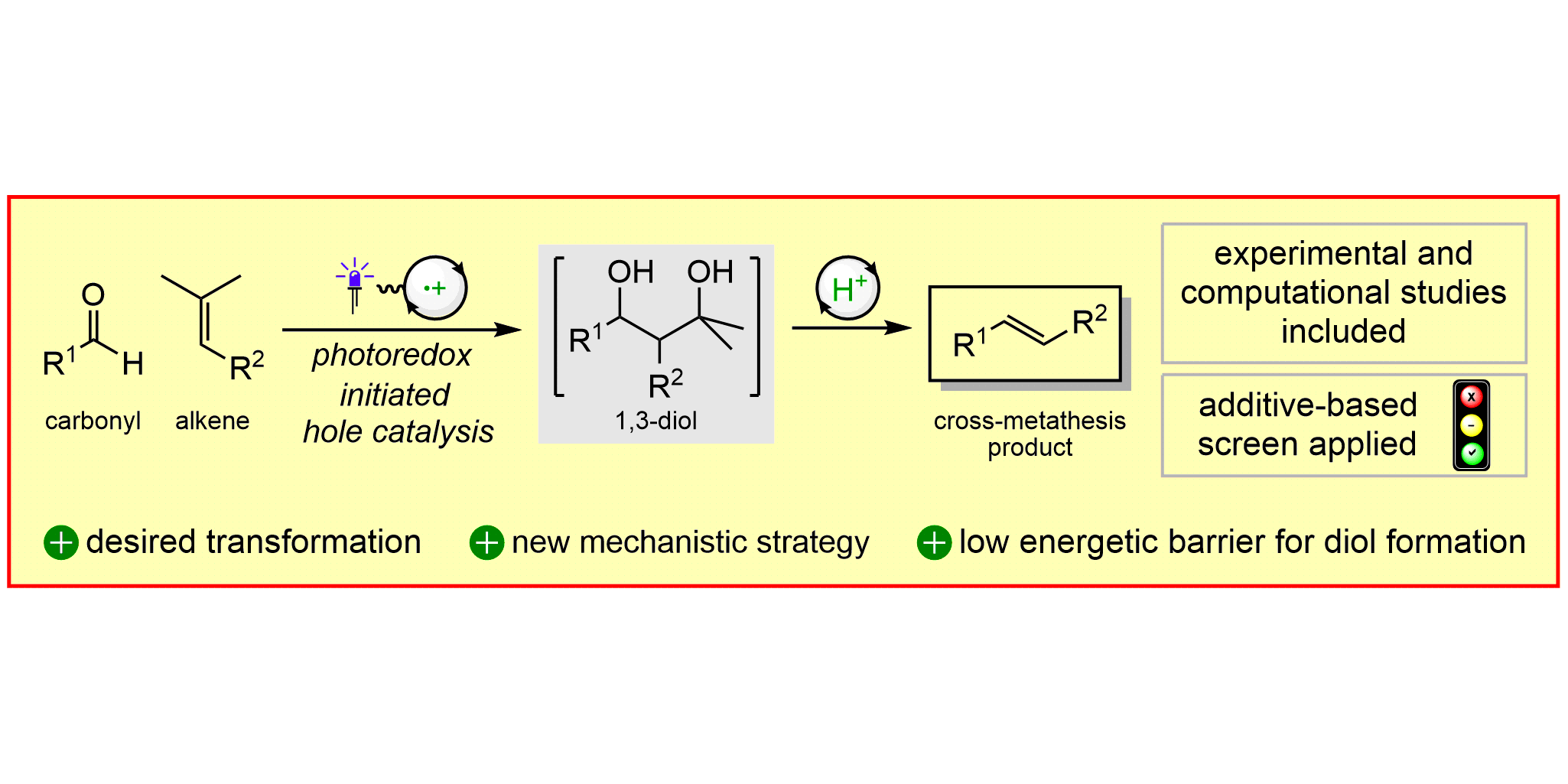
L. Pitzer,§ F. Sandfort,§ F. Strieth-Kalthoff, F. Glorius,
Carbonyl-Olefin Cross-Metathesis Through a Visible-Light-Induced 1,3-Diol Formation and Fragmentation Sequence,
Angew. Chem. Int. Ed. 2018, 57, 16219-16223; Angew. Chem. 2018, 130, 16453-16457.
§ Both authors contributed equally.
J. L. Schwarz,§ F. Schäfers,§ A. Tlahuext-Aca, L. Lückemeier, F. Glorius,
Diastereoselective Allylation of Aldehydes by Dual Photoredox and Chromium Catalysis,
J. Am. Chem. Soc. 2018, 140, 12705-12709.
§ Both authors contributed equally.
F. Strieth-Kalthoff, M. J. James, M. Teders, L. Pitzer, F. Glorius,
Energy transfer catalysis mediated by visible light: principles, applications, directions,
Chem. Soc. Rev. 2018, 47, 7190-7202.
M. Teders, C. Henkel, L. Anhäuser, F. Strieth-Kalthoff, A. Goméz-Suárez, R. Kleinmans, A. Kahnt, A. Rentmeister, D. M. Guldi,* F. Glorius,*
The energy-transfer-enabled biocompatible disulfide–ene reaction,
Nat. Chem. 2018, 10, 981-988.
M. J. James, J. L. Schwarz, F. Strieth-Kalthoff, B. Wibbeling, F. Glorius,
Dearomative Cascade Photocatalysis: Divergent Synthesis through Catalyst Selective Energy Transfer,
J. Am. Chem. Soc. 2018, 140, 8624-8628.
S. Mukherjee, T. Patra, F. Glorius,
Cooperative Catalysis: A Strategy to Synthesize Trifluoromethyl-thioesters from Aldehydes,
ACS Catal. 2018, 8, 5842-5846.
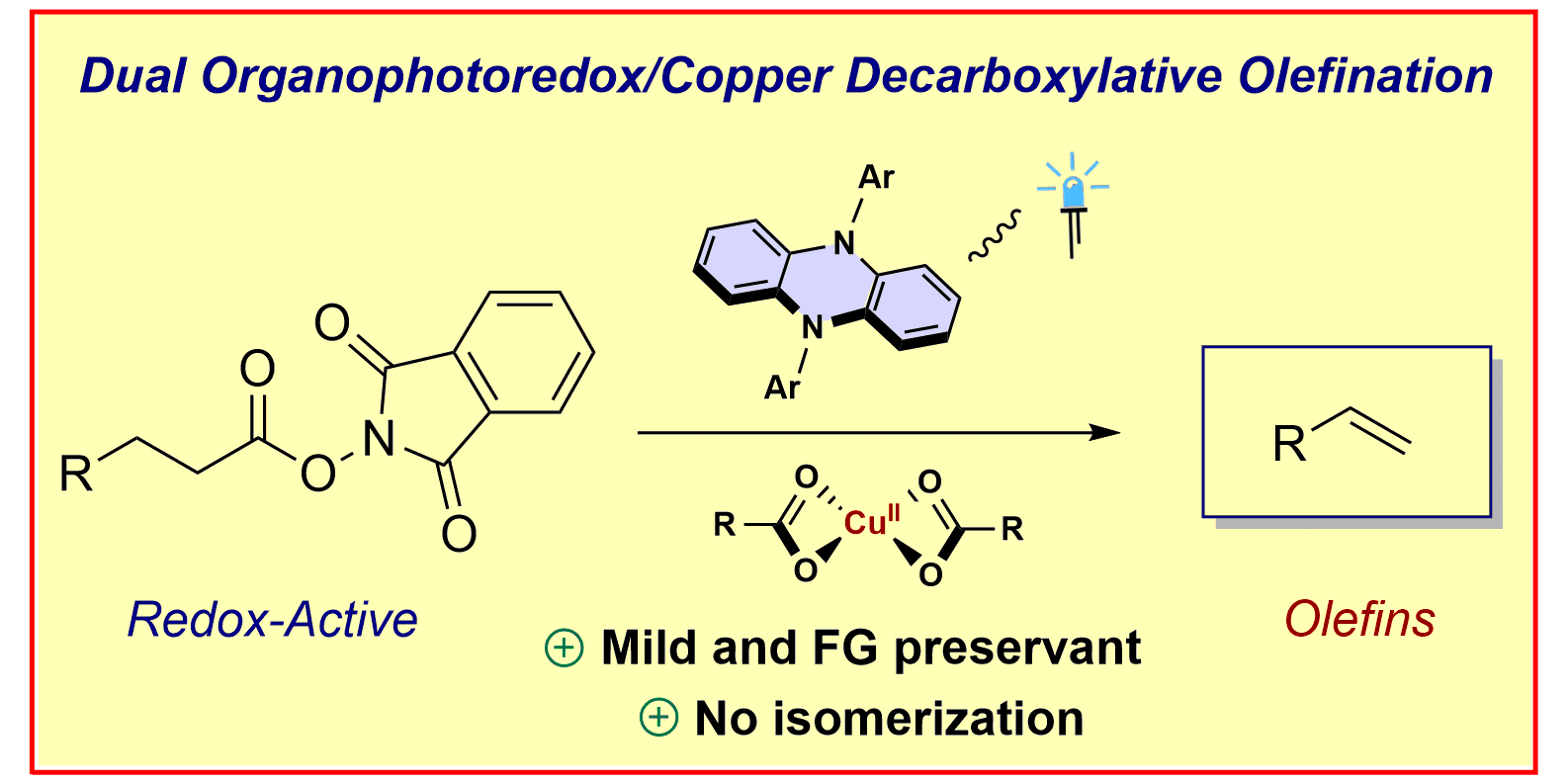
A. Tlahuext-Aca, L. Candish, R. A. Garza-Sanchez, F. Glorius,
Decarboxylative Olefination of Activated Aliphatic Acids Enabled by Dual Organophotoredox/Copper Catalysis,
ACS Catal. 2018, 8, 1715-1719.
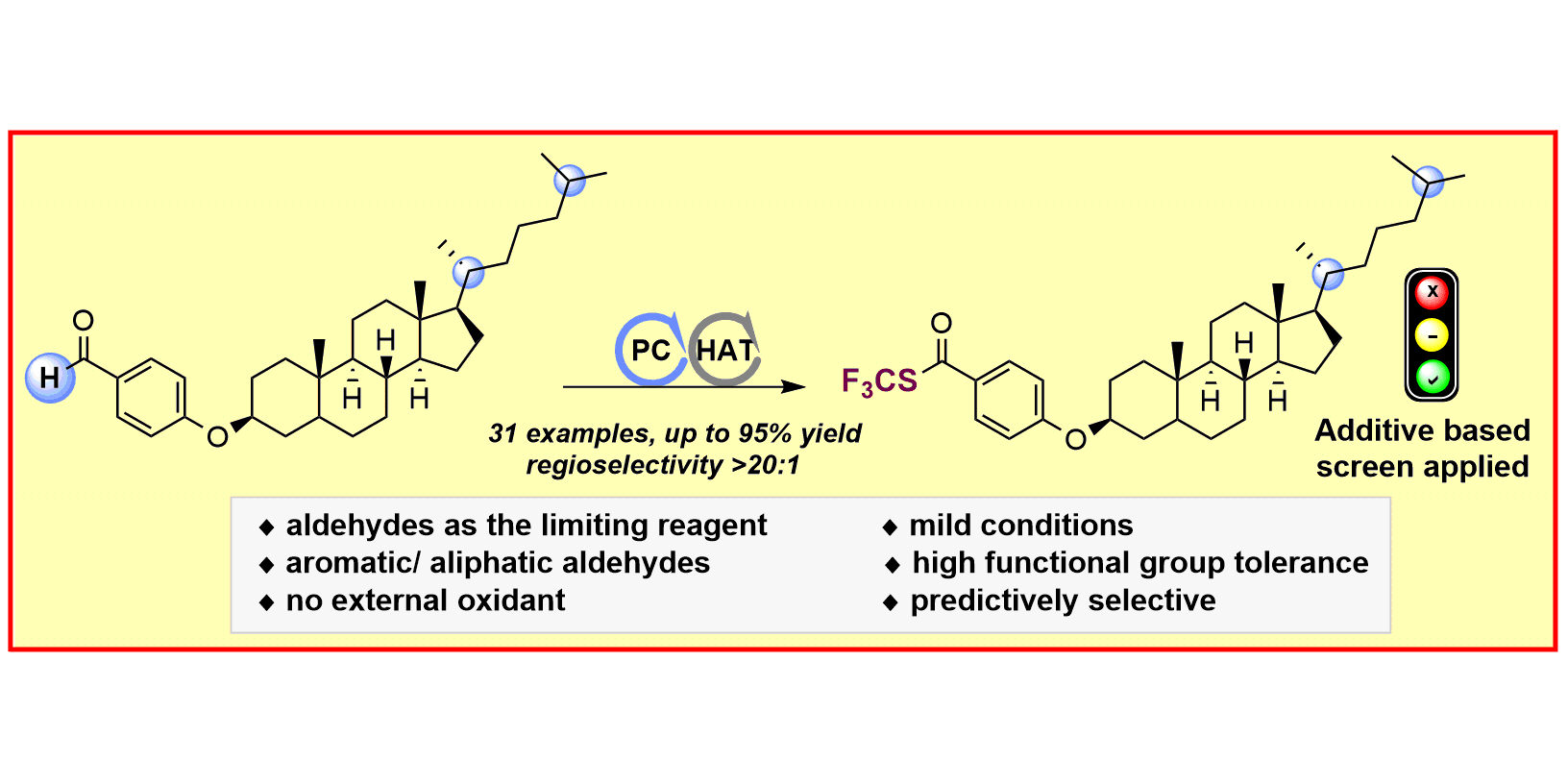
S. Mukherjee, R. A. Garza-Sanchez§, A. Tlahuext-Aca§, F. Glorius,
Alkynylation of Csp2(O)–H Bonds Enabled by Photoredox-Mediated Hydrogen-Atom Transfer,
Angew. Chem. Int. Ed. 2017, 56, 14723-14726; Angew. Chem. 2017, 129, 14915-14919.
§ Both authors contributed equally.
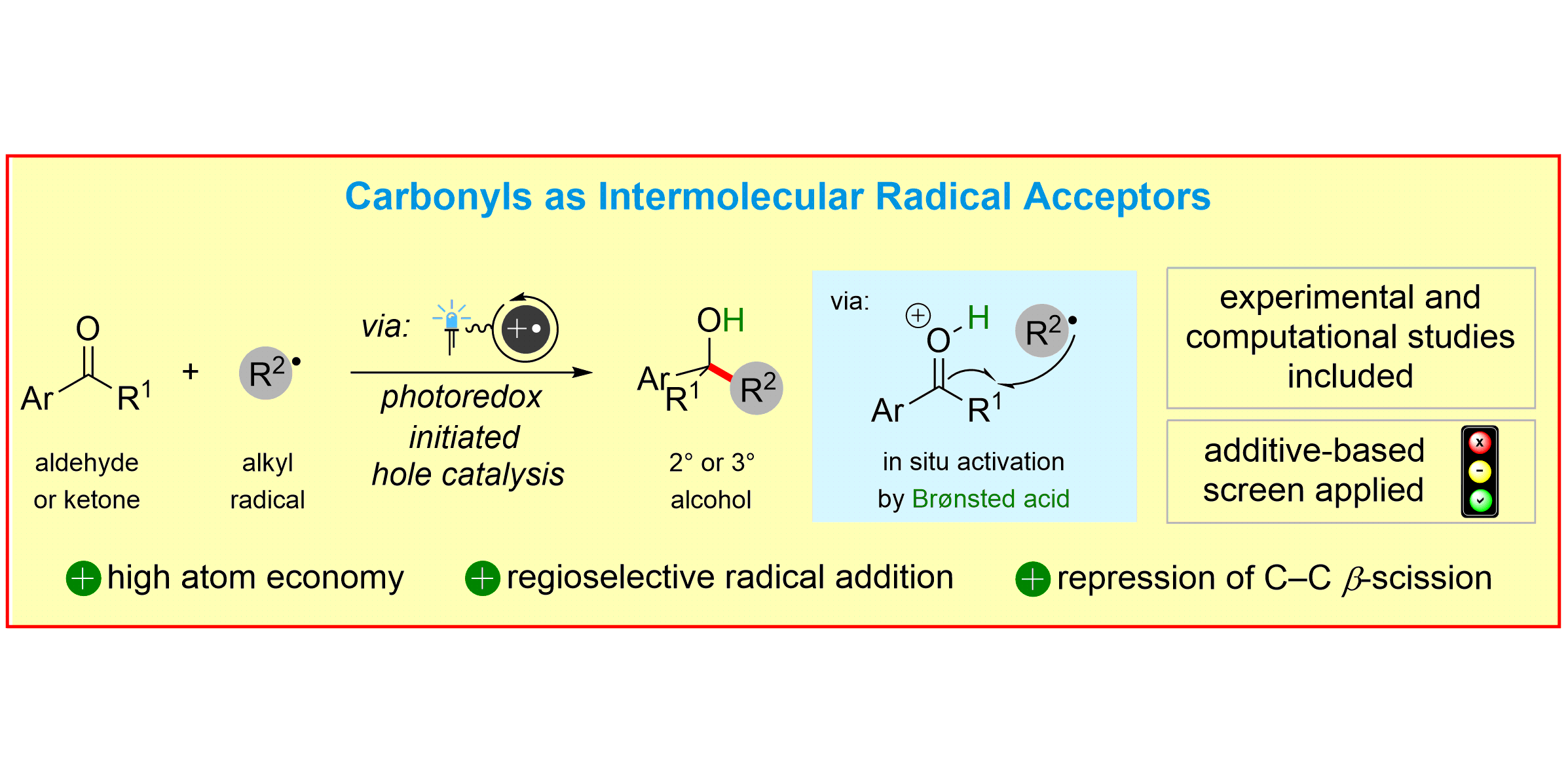
L. Pitzer§, F. Sandfort§, F. Strieth-Kalthoff, F. Glorius,
Intermolecular Radical Addition to Carbonyls Enabled by Visible Light Photoredox Initiated Hole Catalysis,
J. Am. Chem. Soc. 2017, 139, 13652-13655.
§ Both authors contributed equally.
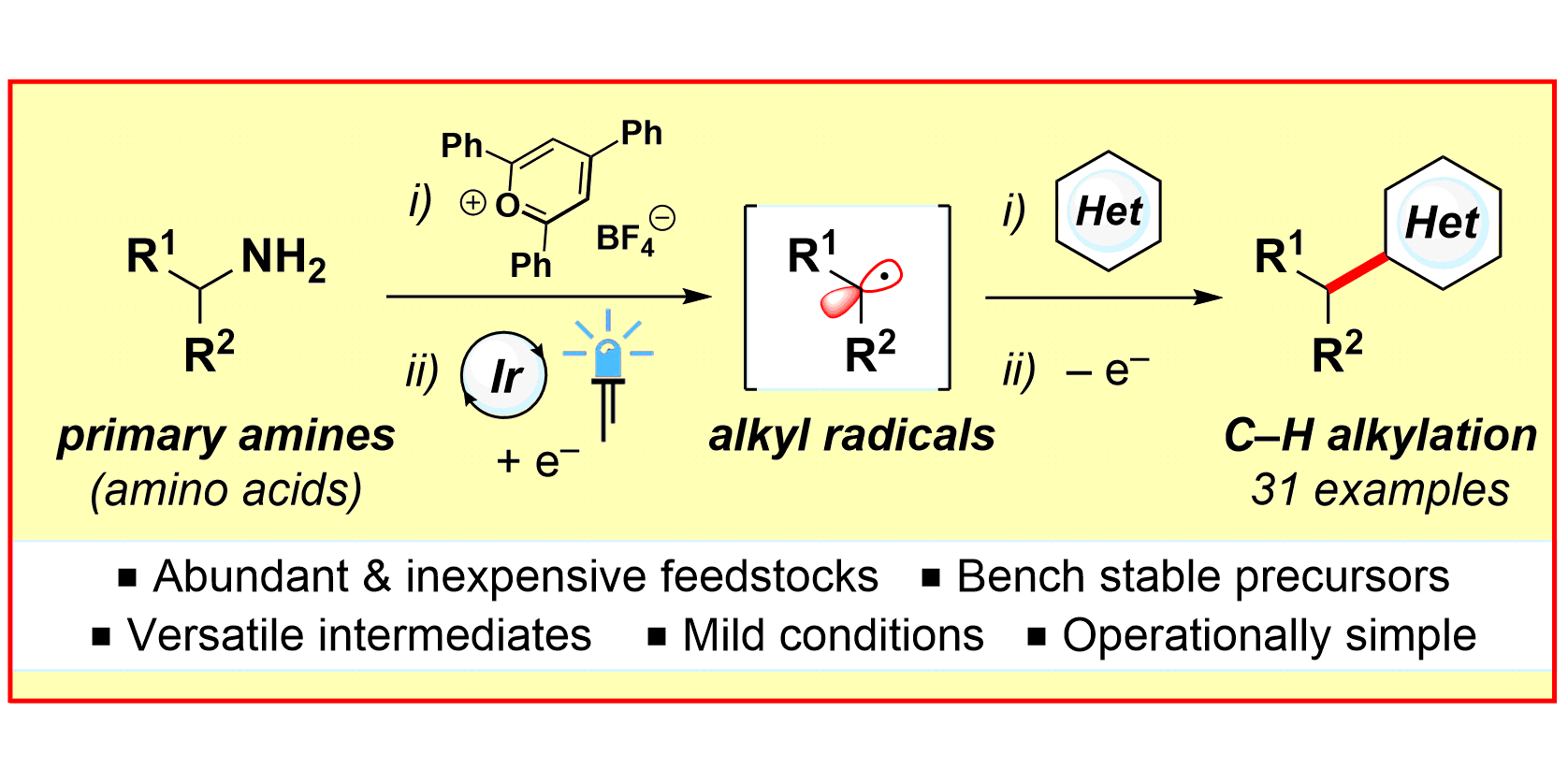
Deaminative Strategy for the Visible Light-Mediated Generation of Alkyl Radicals,
Angew. Chem. Int. Ed. 2017, 56, 12336-12339; Angew. Chem. 2017, 129, 12505-12509.
§ Both authors contributed equally.
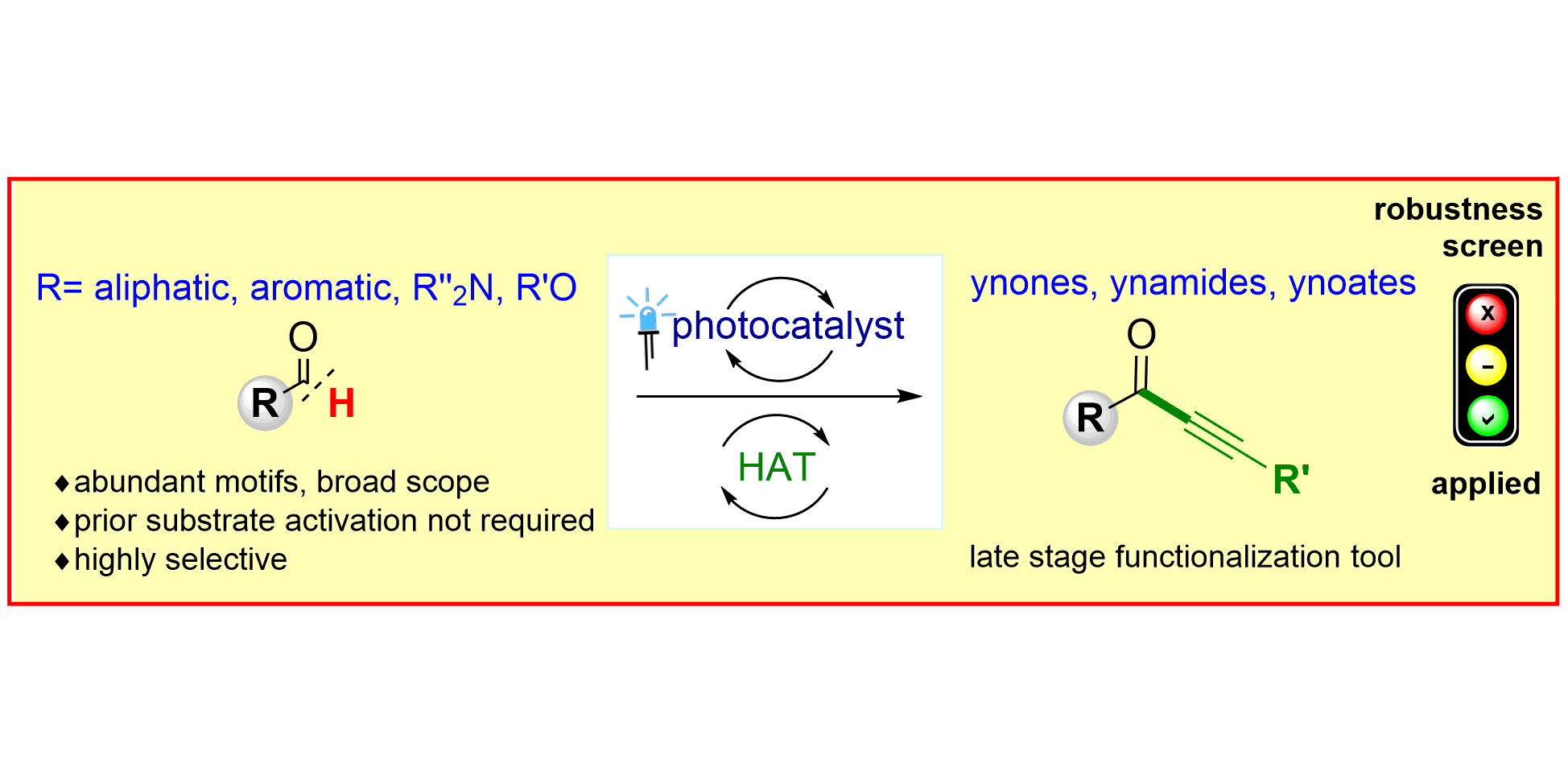
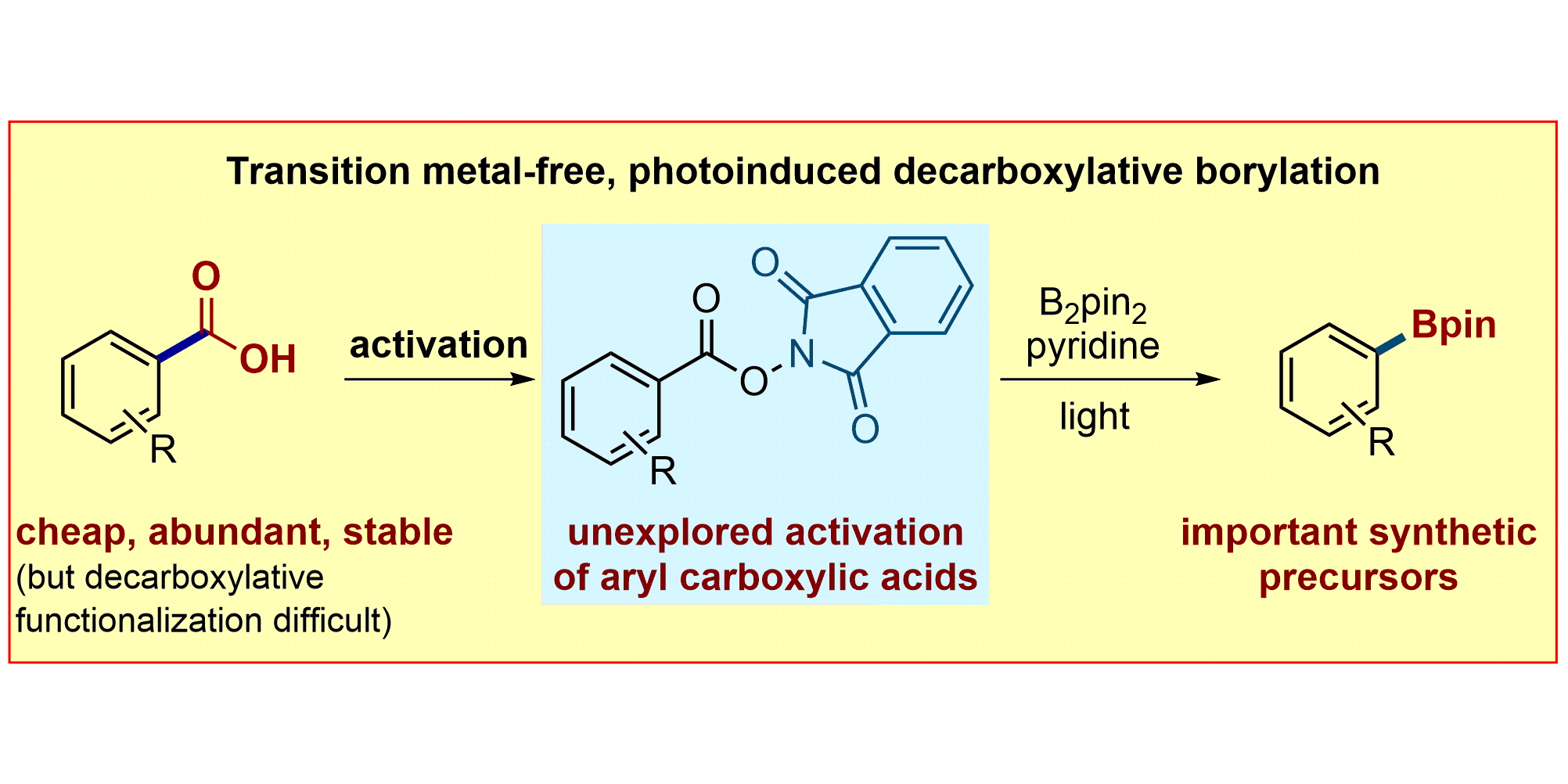
L. Candish, M. Teders, F. Glorius,
Transition-Metal-Free, Visible-Light-Enabled Decarboxylative Borylation of Aryl N‑Hydroxyphthalimide Esters,
J. Am. Chem. Soc. 2017, 139, 7440-7443.
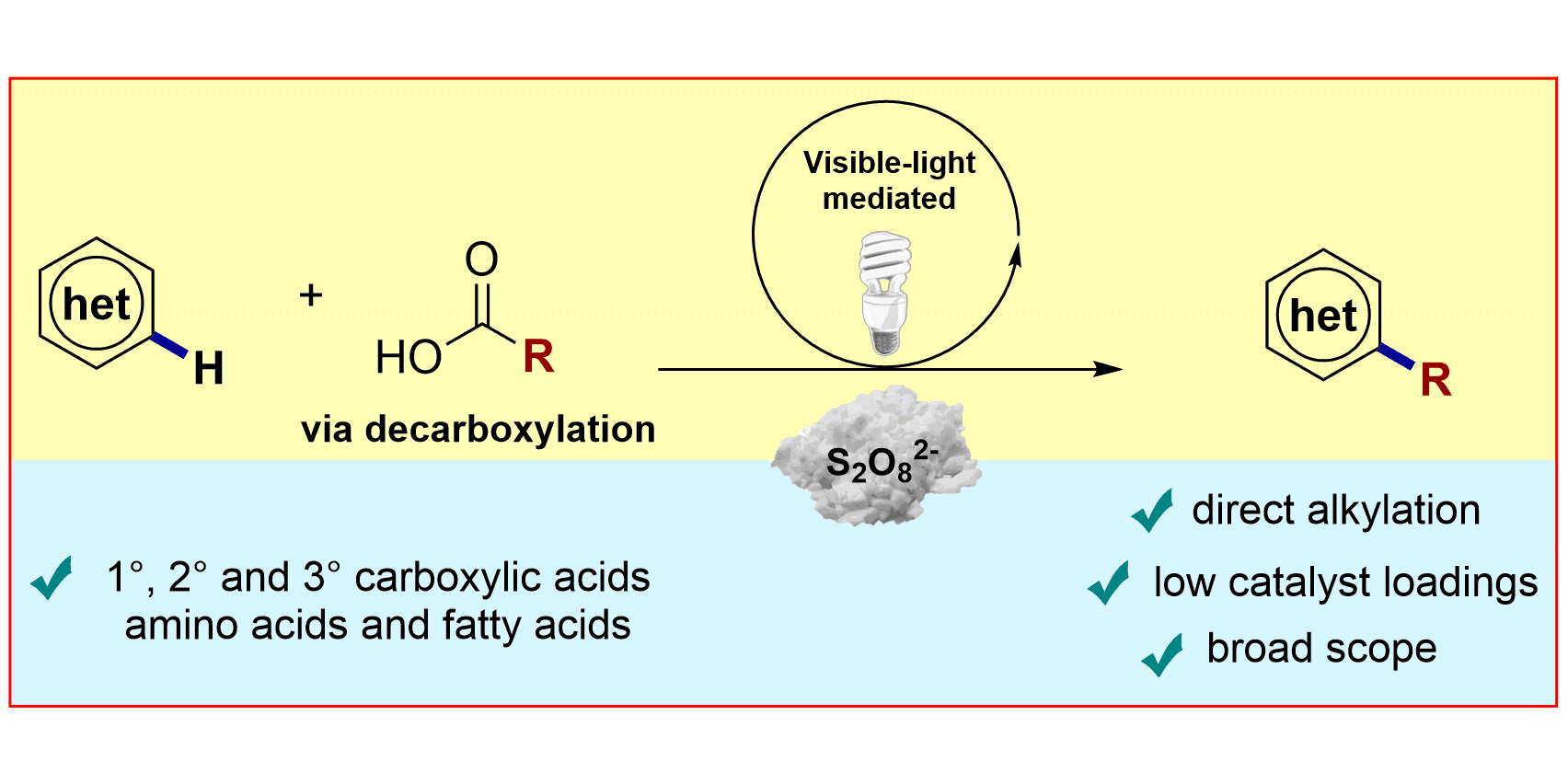
R. A. Garza-Sanchez, A. Tlahuext-Aca, G. Tavakoli, F. Glorius,
Visible-Light-Mediated Direct Decarboxylative C−H Functionalization of Heteroarenes,
ACS Catal. 2017, 7, 4057-4061.
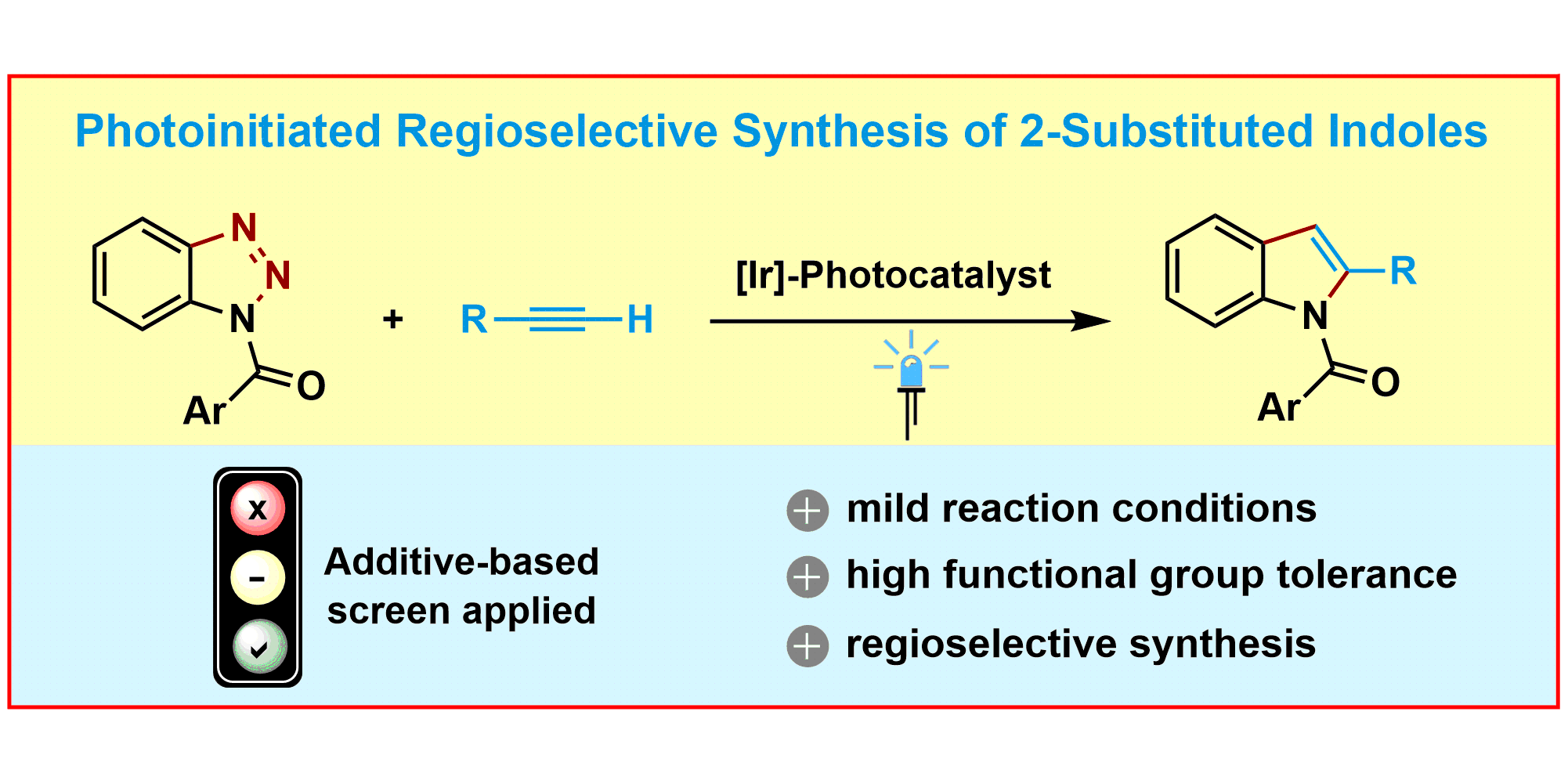
M. Teders, L. Pitzer, S. Buss, F. Glorius,
Regioselective Synthesis of 2‑Substituted Indoles from Benzotriazoles and Alkynes by Photoinitiated Denitrogenation,
ACS Catal. 2017, 7, 4053-4056.
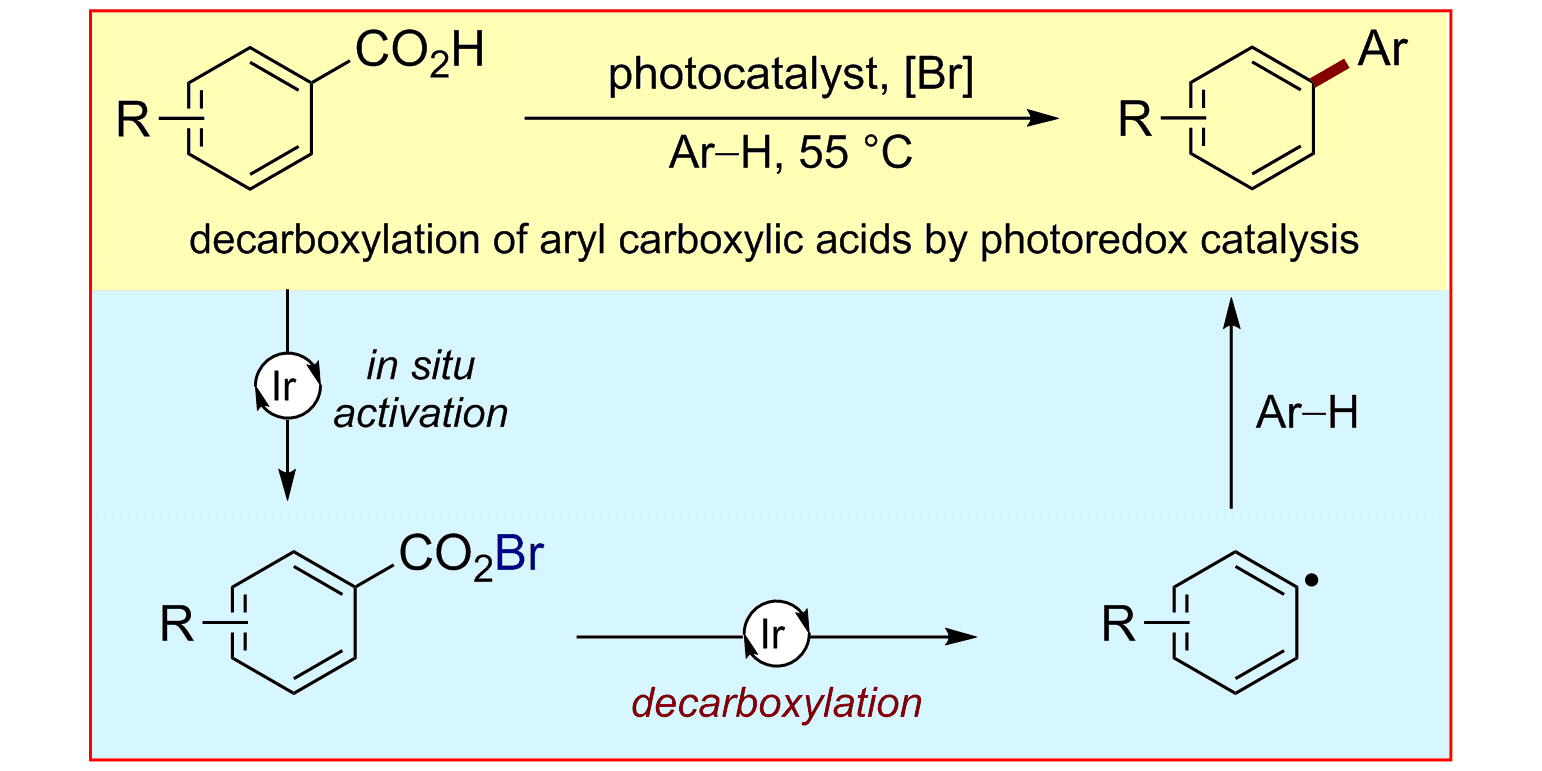
L. Candish, M. Freitag, T. Gensch, F. Glorius,
Mild, visible light-mediated decarboxylation of aryl carboxylic acids to access aryl radicals,
Chem. Sci. 2017, 8, 3618-3622.
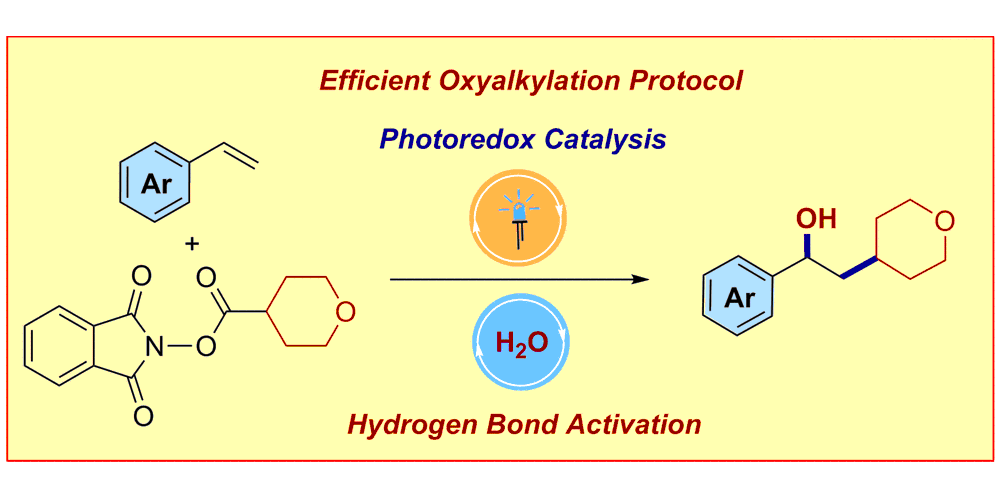
A. Tlahuext-Aca, R. A. Garza-Sanchez, F. Glorius,
Multicomponent Oxyalkylation of Styrenes Enabled by Hydrogen Bond Assisted Photoinduced Electron Transfer,
Angew. Chem. Int. Ed. 2017, 56, 3708-3711; Angew. Chem. 2017,129, 3762-3765.
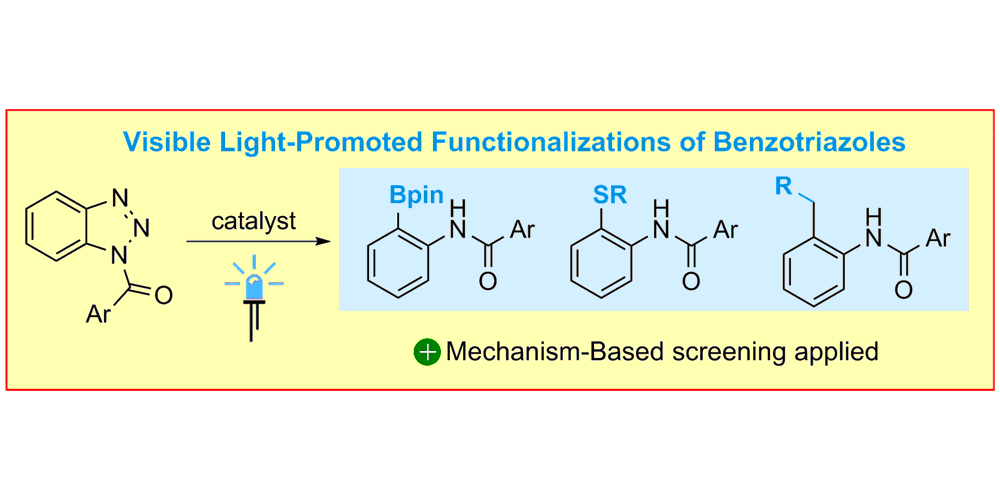
M. Teders, A. Gómez-Suárez§, L. Pitzer§, M. N. Hopkinson, F. Glorius,
Diverse Visible-Light-Promoted Functionalizations of Benzotriazoles Inspired by Mechanism-Based Luminescence Screening,
Angew. Chem. Int. Ed. 2017, 56, 902-906; Angew. Chem. 2017, 129, 921-925.
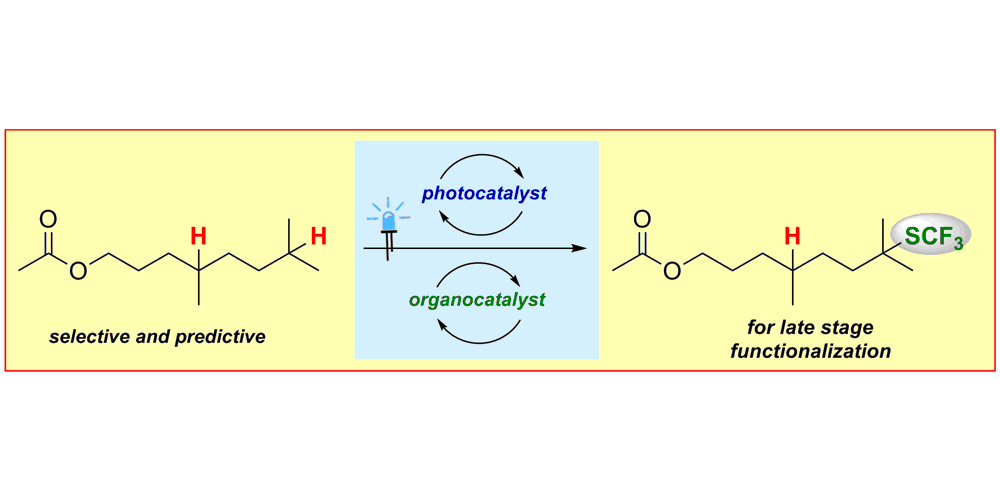
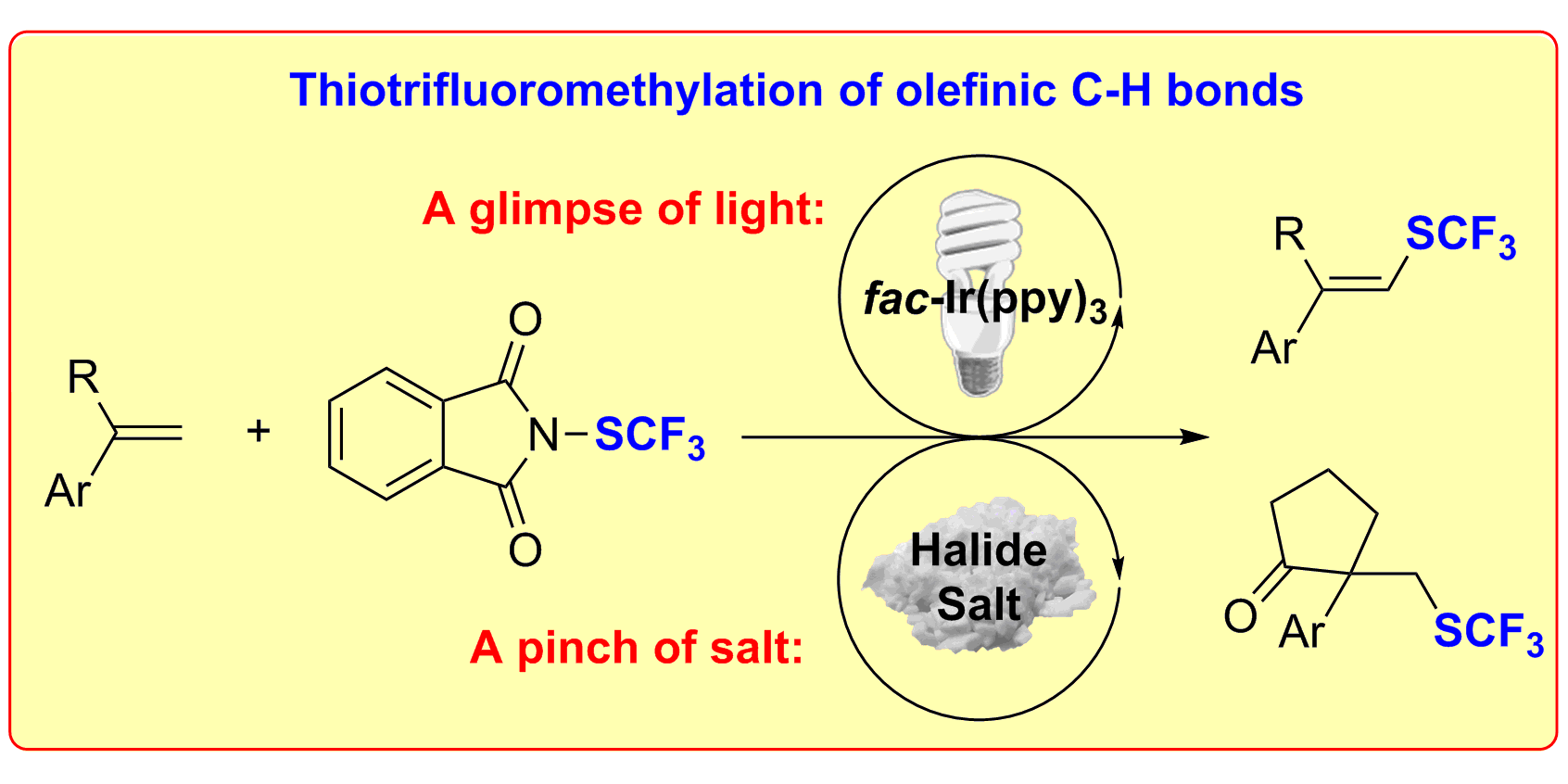
S. Mukherjee,§ B. Maji,§ A. Tlahuext-Aca, F. Glorius,
Visible-Light-Promoted Activation of Unactivated C(sp3)−H Bonds and Their Selective Trifluoromethylthiolation,
J. Am. Chem. Soc. 2016, 138, 16200-16203.
§ Both authors contributed equally.
M. N. Hopkinson, A. Tlahuext-Aca, F. Glorius,
Merging Visible Light Photoredox and Gold Catalysis,
Acc. Chem. Res. 2016, 49, 2261-2272.
Published as part of the Accounts of Chemical Research special issue “Photoredox Catalysis in Organic Chemistry”
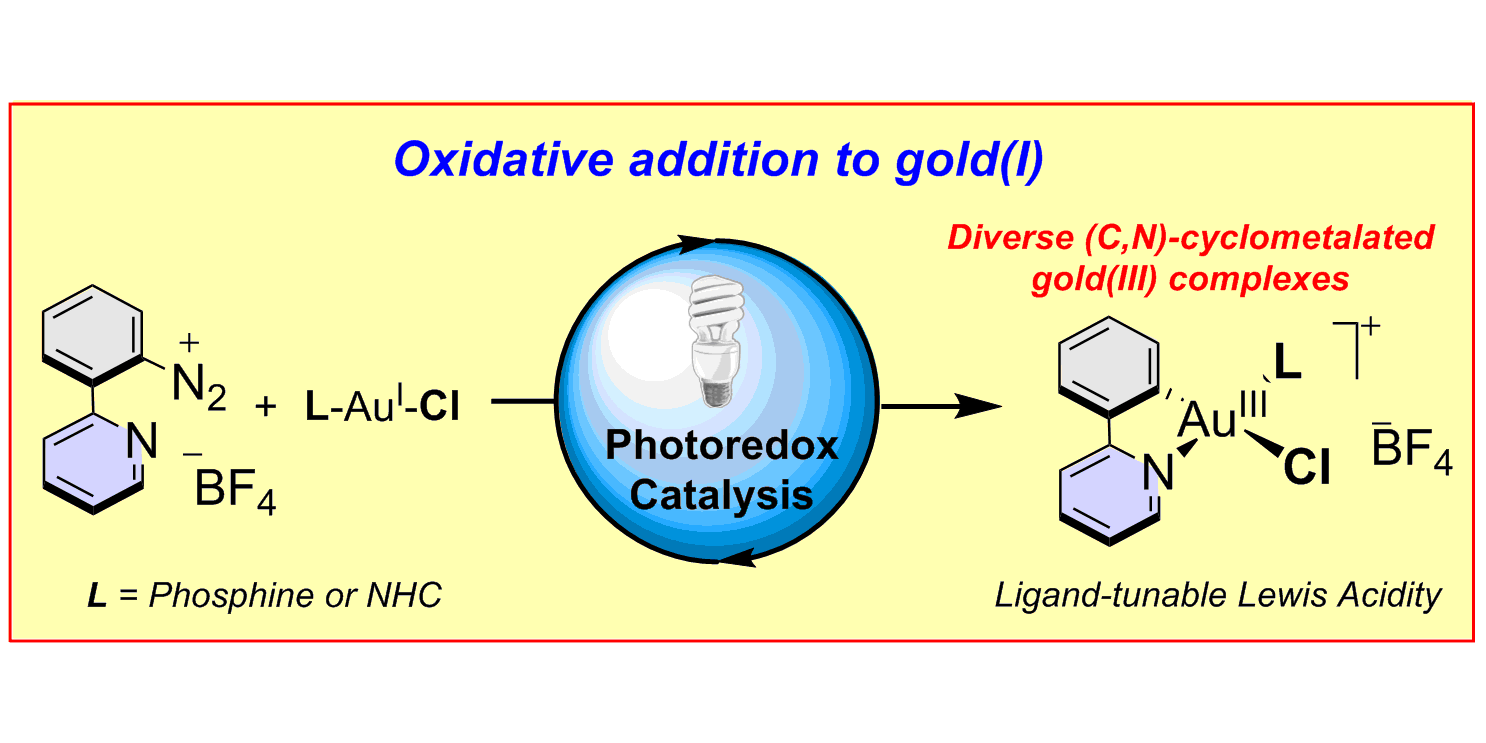
A. Tlahuext-Aca, M. N. Hopkinson, C. G. Daniliuc, F. Glorius,
Oxidative Addition to Gold(I) by Photoredox Catalysis: Straightforward Access to Diverse (C,N)-Cyclometalated Gold(III) Complexes,
Chem. Eur. J. 2016, 22, 11587-11592.
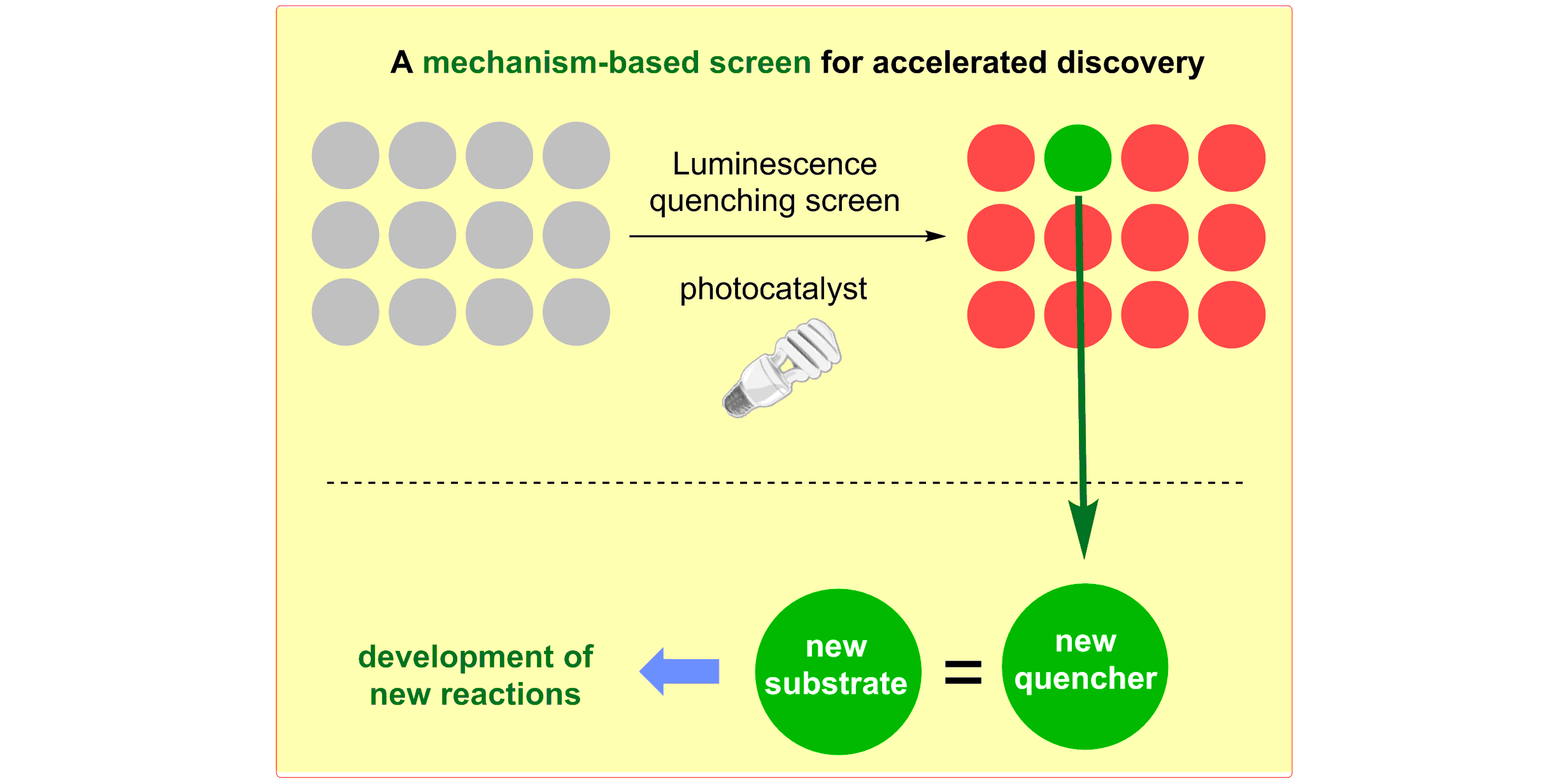
M. N. Hopkinson, A. Gomez-Suarez, M. Teders, B. Sahoo, F. Glorius,
Accelerated Discovery in Photocatalysis using a Mechanism-Based Screening Method,
Angew. Chem. Int. Ed. 2016, 55, 4361-4366; Angew. Chem. 2016, 128, 4434-4439.
This paper was selected for the Angewandte front cover image of the issue it appears in. Please, enjoy this "Catalyst Speed Dating!"
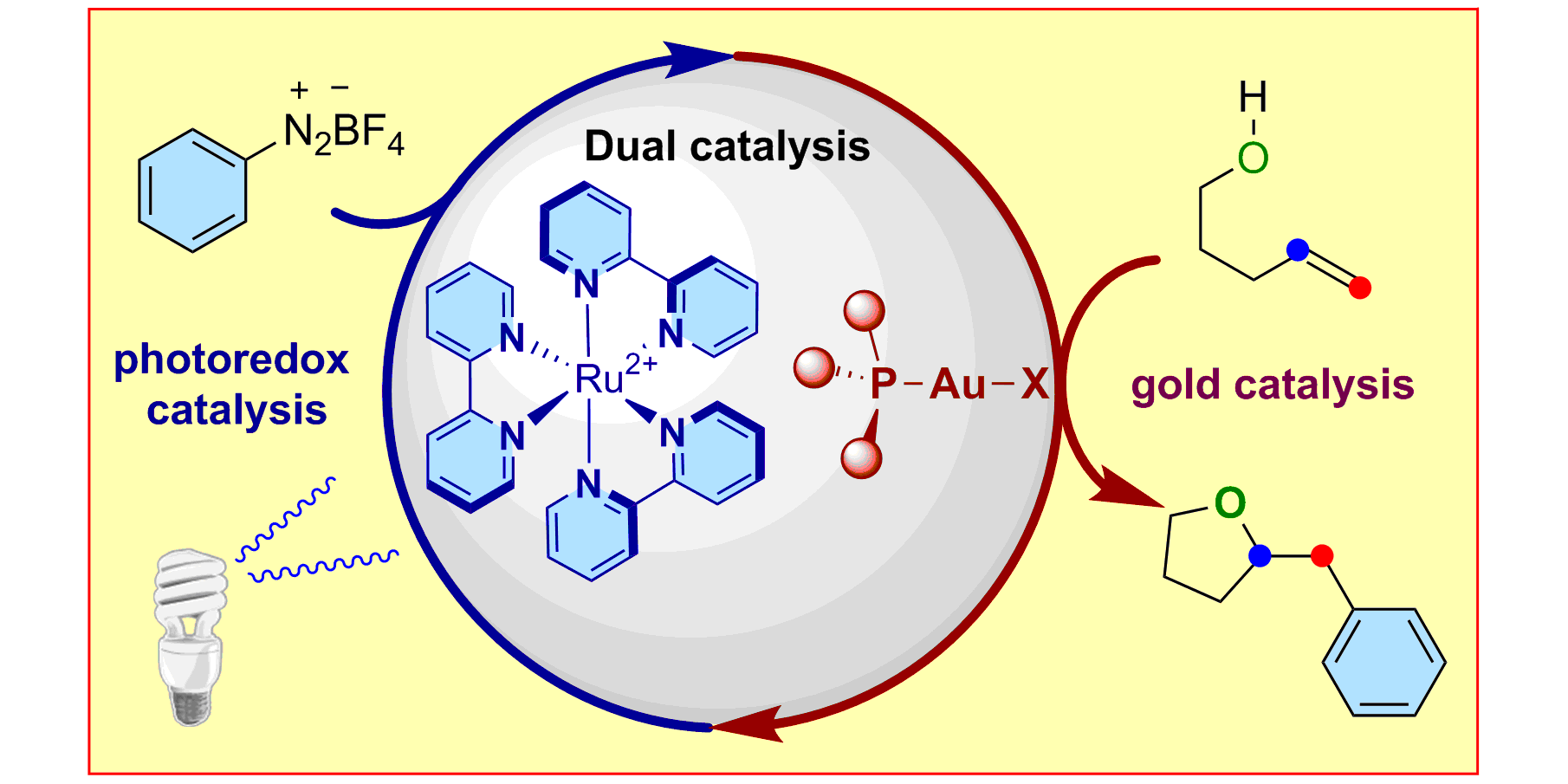
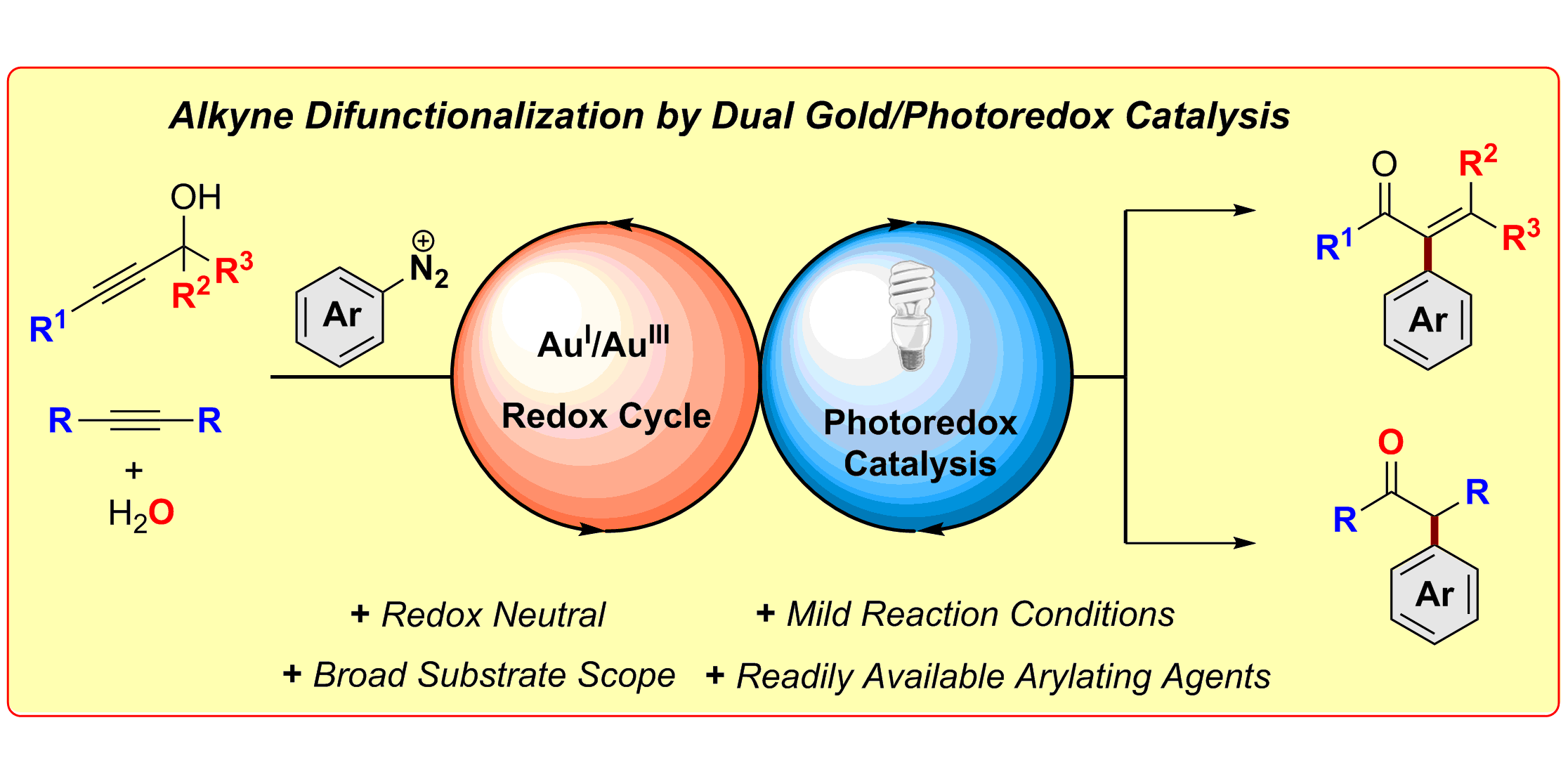
Alkyne Difunctionalization by Dual Gold/Photoredox Catalysis,
Chem. Eur. J. 2016, 22, 5909-5913.
§ Both authors contributed equally.
L. Candish,§ L. Pitzer,§ A. Gomez-Suarez, F. Glorius,
Visible Light-Promoted Decarboxylative Di- and Trifluoromethylthiolation of Alkyl Carboxylic Acids,
Chem. Eur. J. 2016, 22, 4753-4756.
§ Both authors contributed equally.
R. Honeker, R. A. Garza-Sanchez, M. N. Hopkinson,* F. Glorius,*
Visible Light-Promoted Trifluoromethylthiolation of Styrenes via Dual Photoredox/Halide Catalysis,
Chem. Eur. J. 2016, 22, 4395-4399.
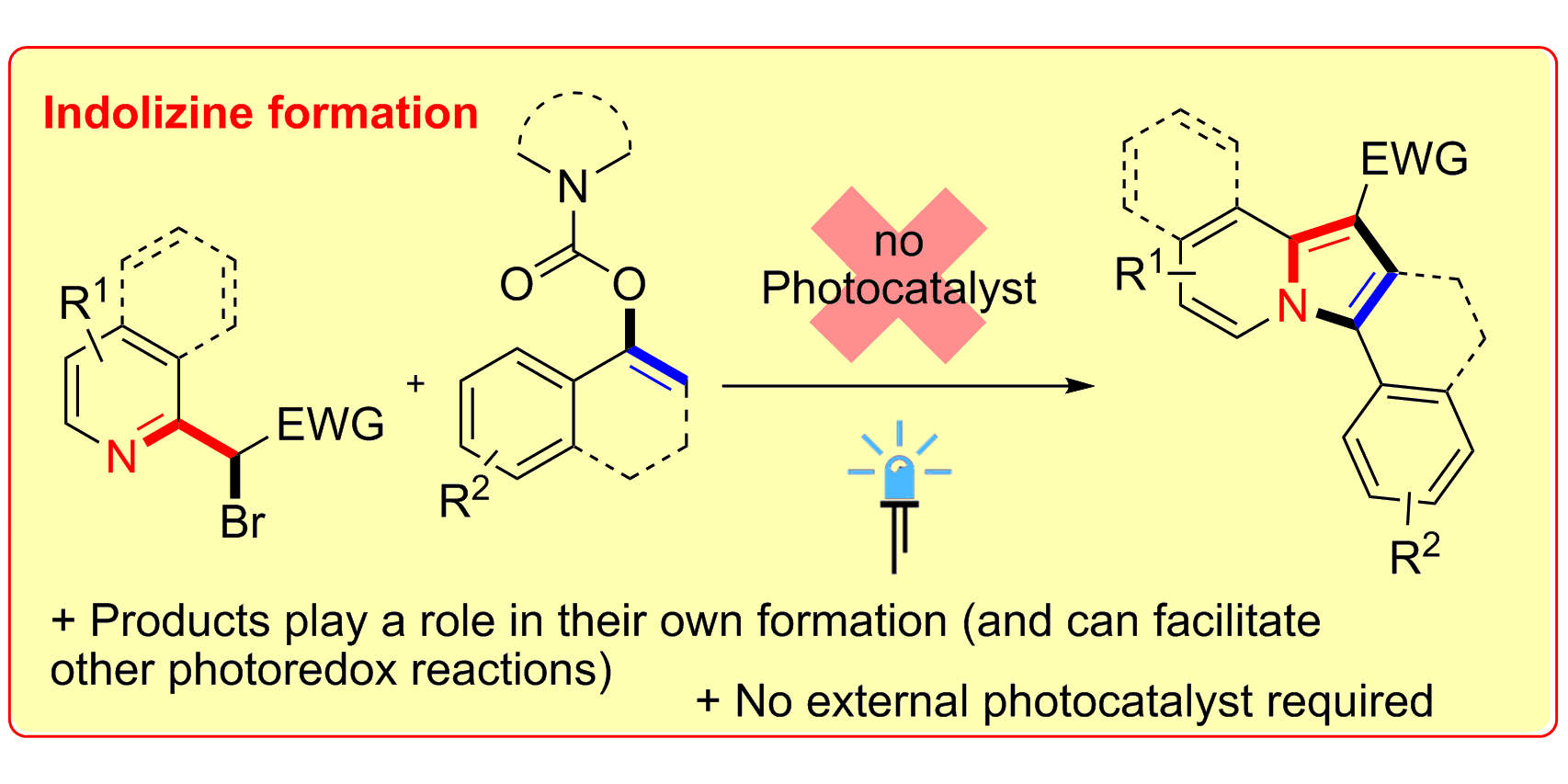
B. Sahoo§, M. N. Hopkinson§, F. Glorius,
External-Photocatalyst-Free Visible-Light-Mediated Synthesis of Indolizines,
Angew. Chem. Int. Ed. 2015, 54, 15545-15549; Angew. Chem. 2015, 127, 15766-15770.
§ Both authors contributed equally.
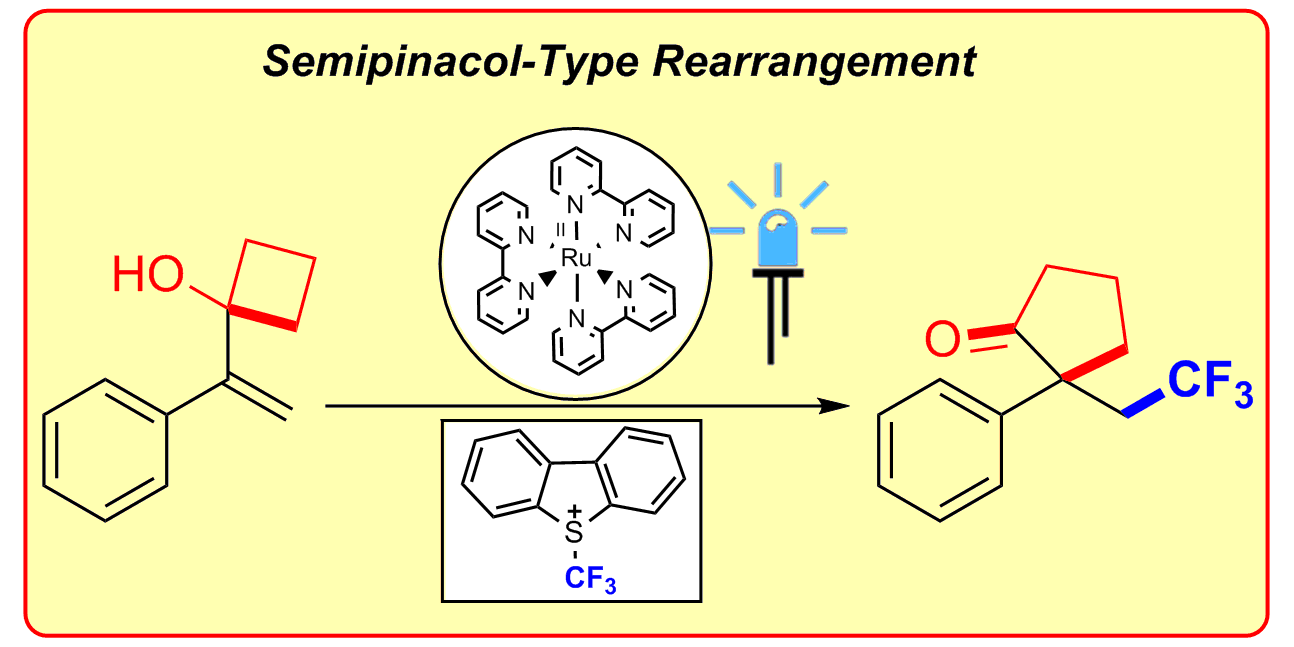
B. Sahoo, J.-L. Li, F. Glorius,
Visible Light Photoredox Catalyzed Semipinacol-Type Rearrangement: Trifluoromethylation/Ring Expansion via a Radical-Polar Mechanism,
Angew. Chem. Int. Ed. 2015, 54, 11577-11580; Angew. Chem. 2015, 127, 11740-11744.
M. N. Hopkinson, B. Sahoo, F. Glorius,
Dual Photoredox and Gold Catalysis: Intermolecular Multicomponent Oxyarylation of Alkenes,
Adv. Synth. Catal. 2014, 356, 2794-2800.
M. N. Hopkinson§, B. Sahoo§, J.-L. Li, F. Glorius,
Dual Catalysis sees the Light: Combining Photoredox with Organo-, Acid and Transition Metal Catalysis,
Chem. Eur. J. 2014, 20, 3874-3886.
§ Both authors contributed equally.
B. Sahoo, M. N. Hopkinson, F. Glorius,
Combining Gold and Photoredox Catalysis: Visible Light-Mediated Oxy- and Aminoarylation of Alkenes,
J. Am. Chem. Soc. 2013, 135, 5505–5508.