Organocatalysis
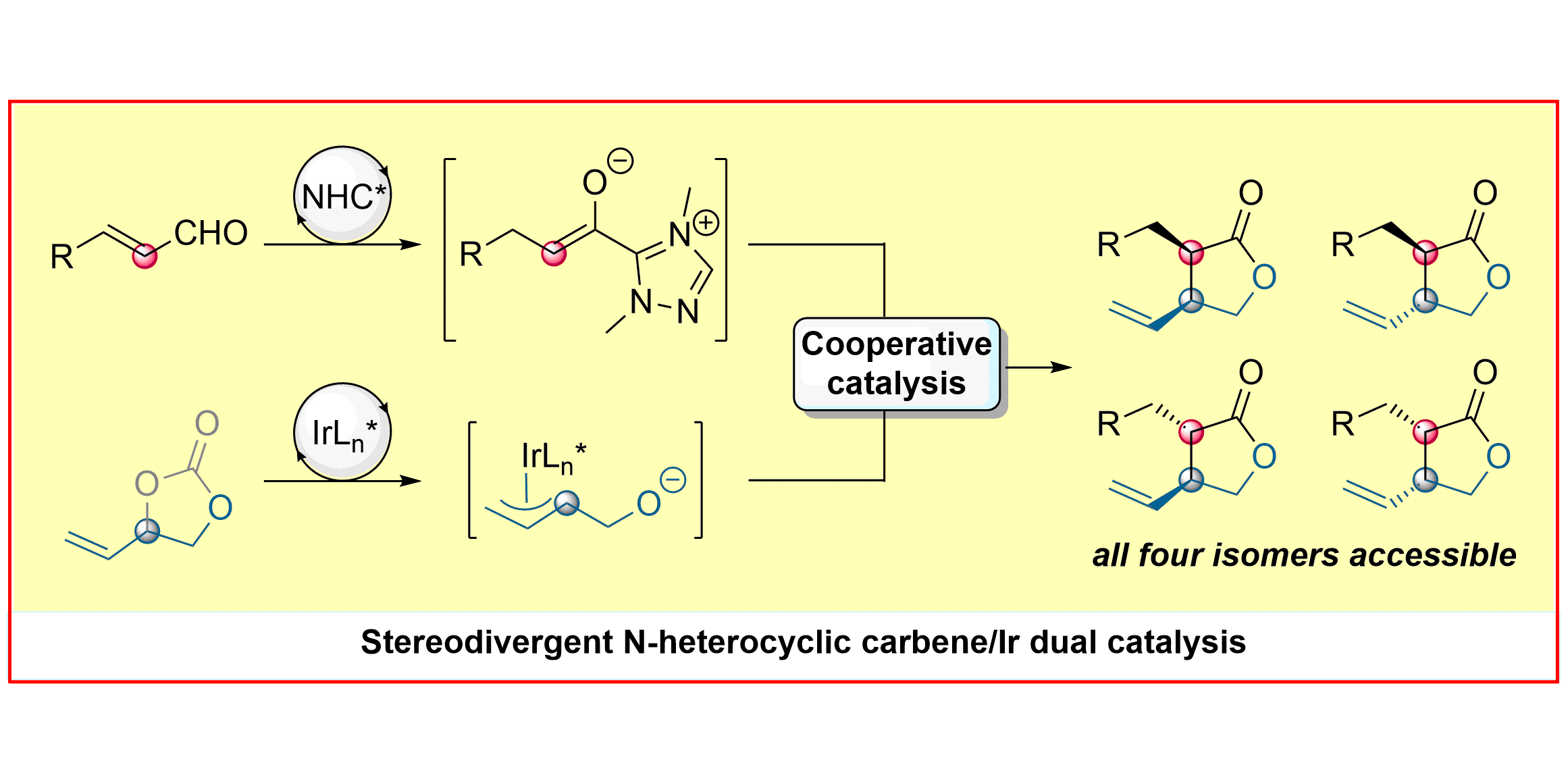
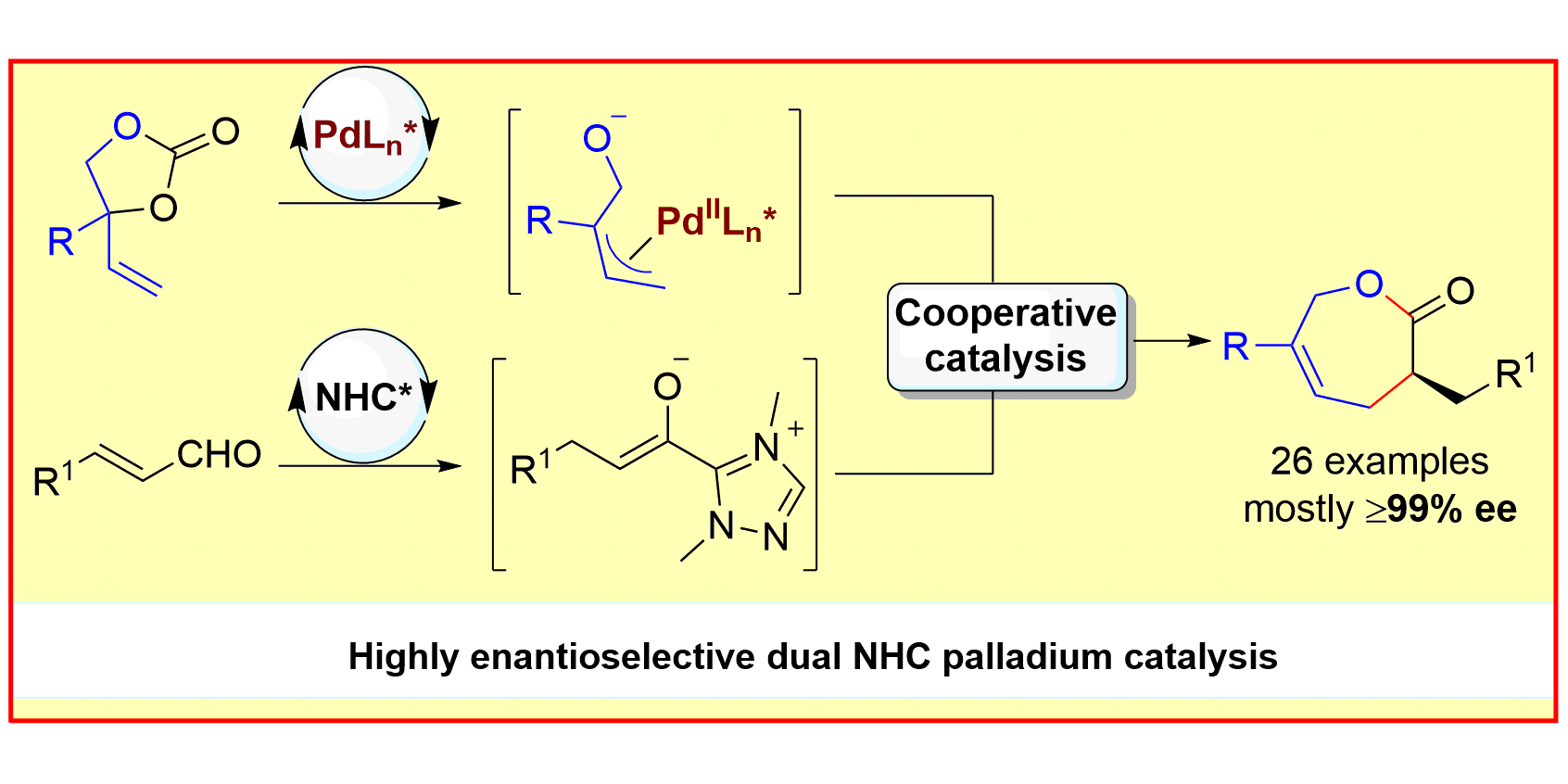
N-heterocyclic carbene (NHC) organocatalysis is a versatile tool for the construction of organic molecules with rich stereochemistry via polarity reversal (umpolung) strategies. Its recent combination with a second cooperative catalytic cycle enables the use of a broader range of reaction partners and provides access to new transformations.[1] Our group has developed novel transformations that merge NHC-bound nucleophiles with electrophilic species generated via transition-metal catalytic cycles to afford valuable organic scaffolds. Depending on the choice of the metal, either 7- or 5-membered lactones can be generated with excellent diastereo- and enantioselectivity.[2] In the case of Ir, a stereodivergent transformation giving access to all four stereoisomers of the α,β-disubstituted-γ-butyrolactones could be achieved.[3]
[1] M. H. Wang, K. A. Scheidt, Angew. Chem. Int. Ed. 2016, 55, 14912. [2] S. Singha, T. Patra, C. G. Daniliuc, F. Glorius, J. Am. Chem. Soc. 2018, 140, 3551. [3] S. Singha, E. Serrano, S. Mondal, C. G. Daniliuc, F. Glorius, Nat. Catal. 2020, 3, 48.
Please click on the graphical abstracts to come to the original publication
S. Singha,§ E. Serrano,§ S. Mondal, C. G. Daniliuc, F. Glorius,
Diastereodivergent synthesis of enantioenriched α,β-disubstituted-γ-butyrolactones via cooperative N-heterocyclic carbene/Ir catalysis,
Nature Catalysis 2020, 3, 48-54.
§ Both authors contributed equally.
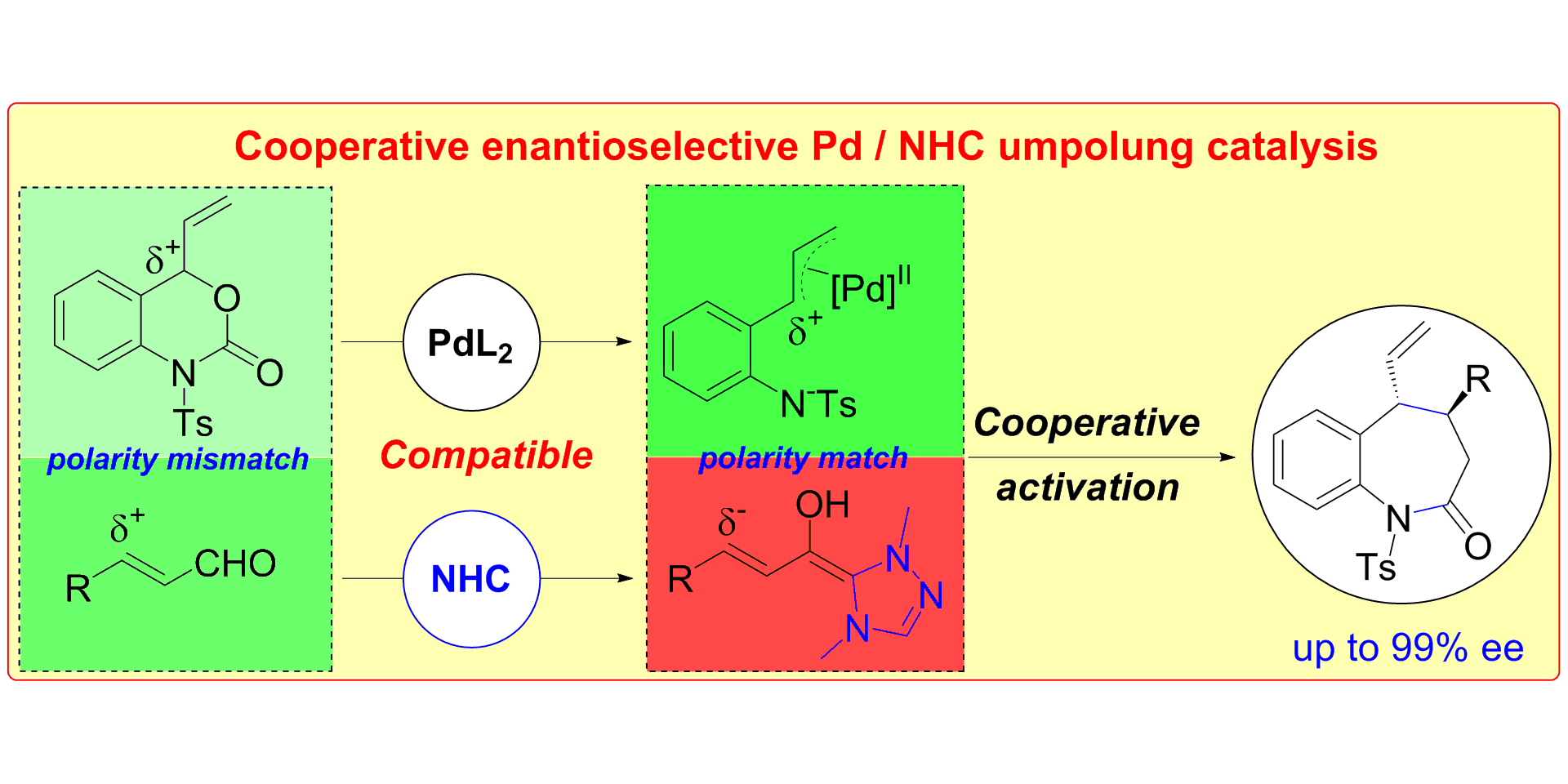
S. Singha, T. Patra, C. G. Daniliuc, F. Glorius,
Highly Enantioselective [5+2] Annulations through Cooperative NHC Organocatalysis and Palladium Catalysis,
J. Am. Chem. Soc. 2018, 140, 3551-3554.
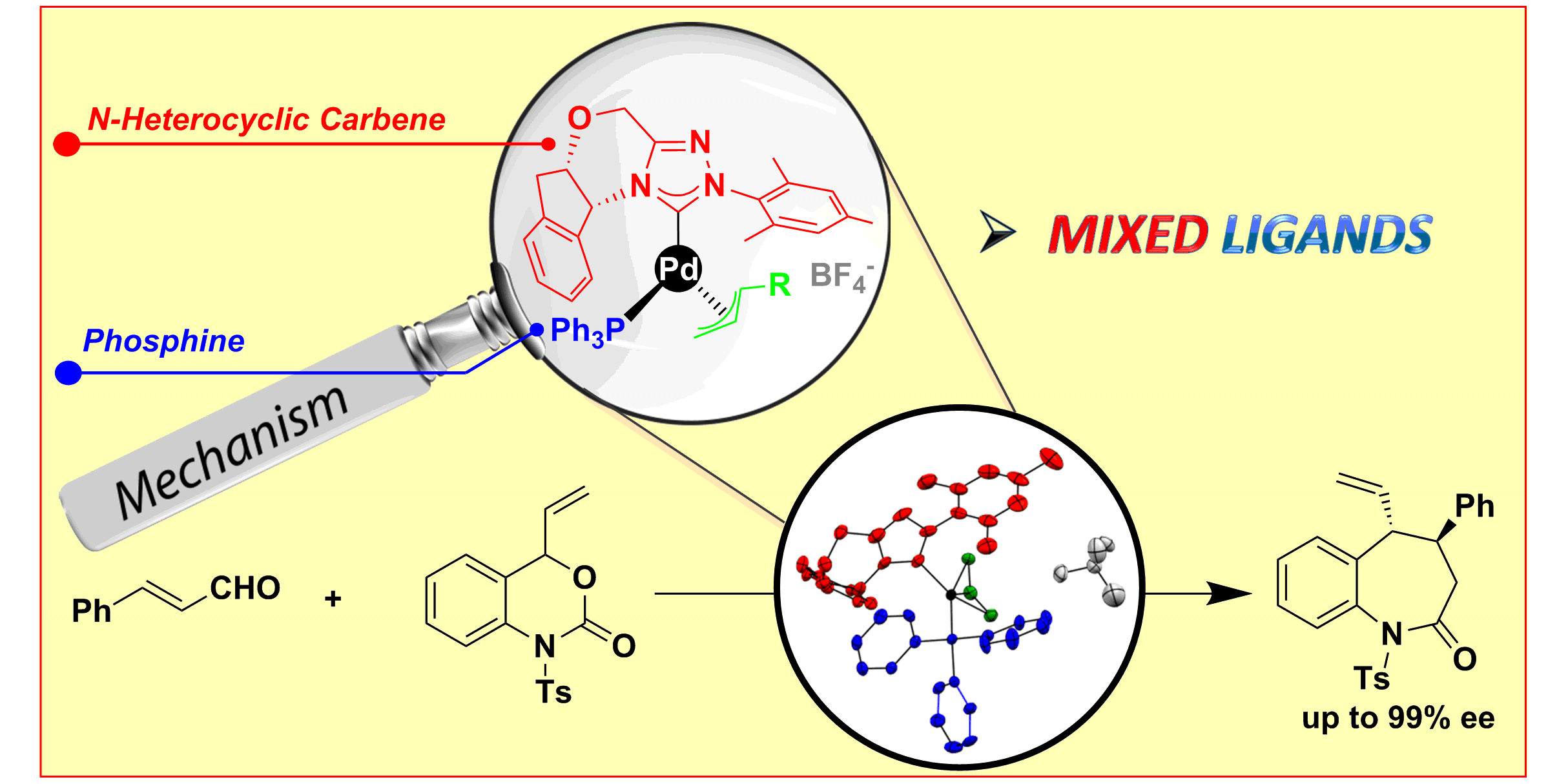
C. Guo,* D. Janssen-Müller, M. Fleige, A. Lerchen, C. G. Daniliuc, F. Glorius,*
Mechanistic Studies on a Cooperative NHC Organocatalysis/Palladium Catalysis System: Uncovering Significant Lessons for Mixed Chiral Pd(NHC)(PR3) Catalyst Design,
J. Am. Chem. Soc. 2017, 139, 4443-4451.
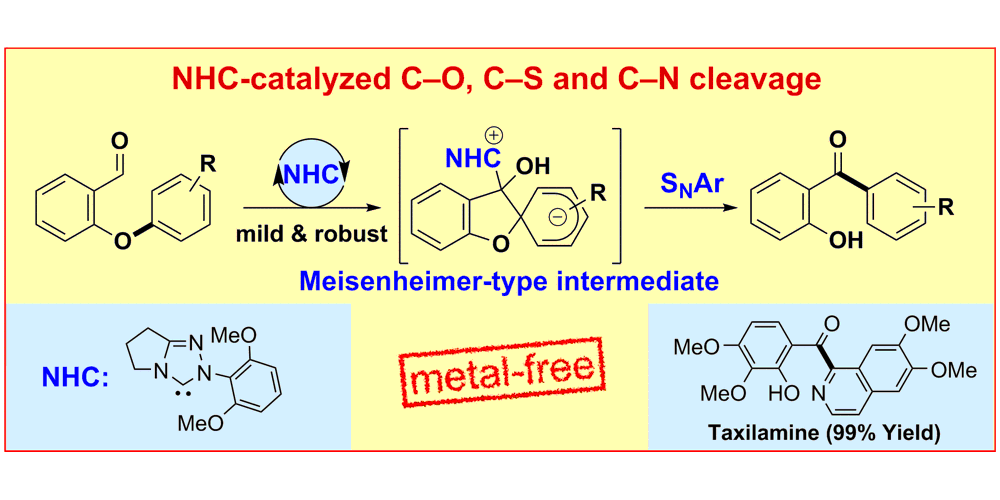
D. Janssen-Müller,§ S. Singha,§ F. Lied, K. Gottschalk, F. Glorius,
NHC-Organocatalyzed CAr–O Bond Cleavage: Mild access to 2 Hydroxybenzophenones,
Angew. Chem. Int. Ed. 2017, 56, 6276-6279; Angew. Chem. 2017, 129, 6373-6376.
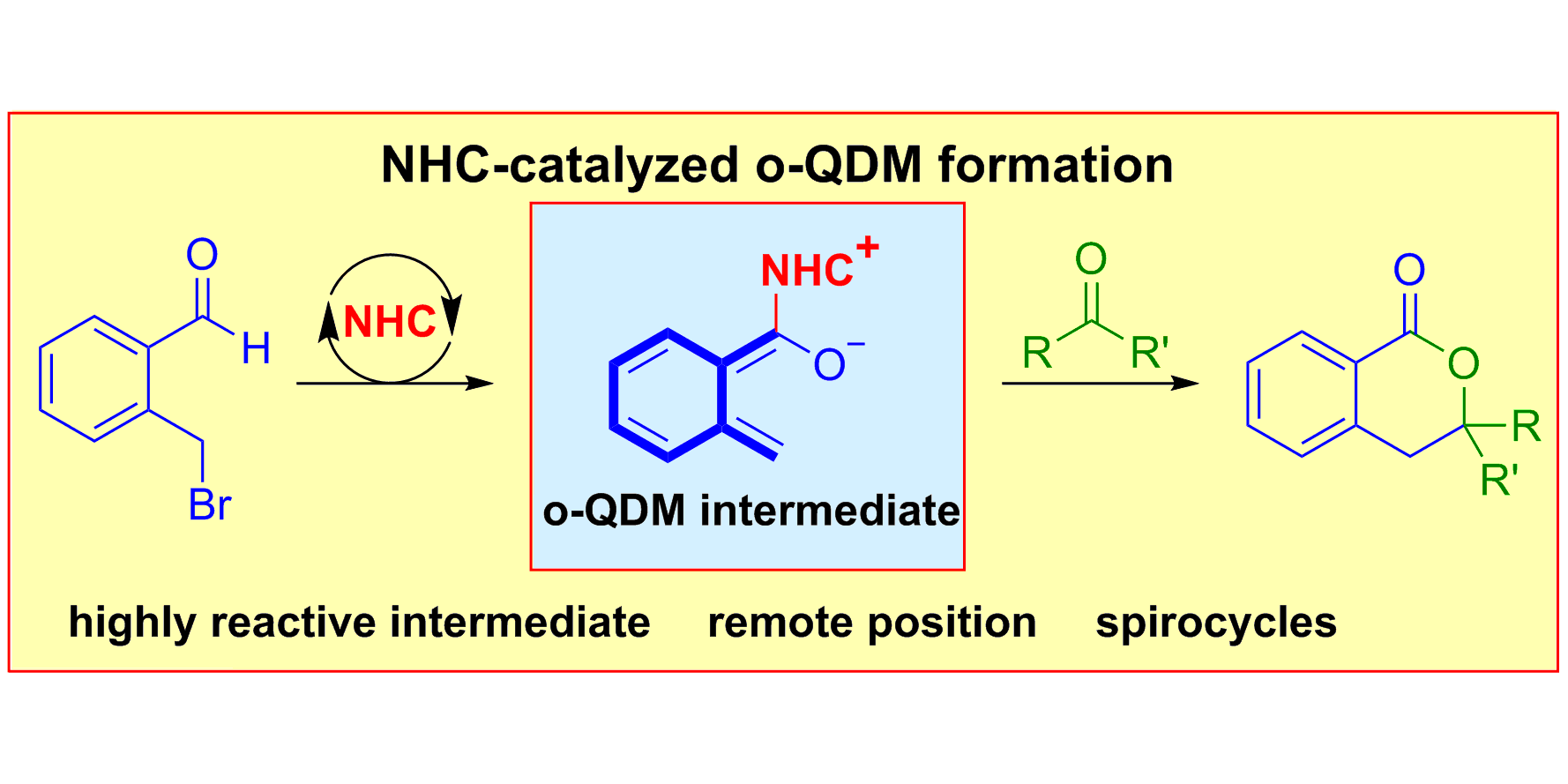
D. Janssen-Müller, S. Singha, T. Olyschläger, C. G. Daniliuc, F. Glorius,
Annulation of o-Quinodimethanes (o-QDM) through NHC-Catalysis for the Synthesis of 1-Isochromanones,
Org. Lett. 2016, 18, 4444-4447.
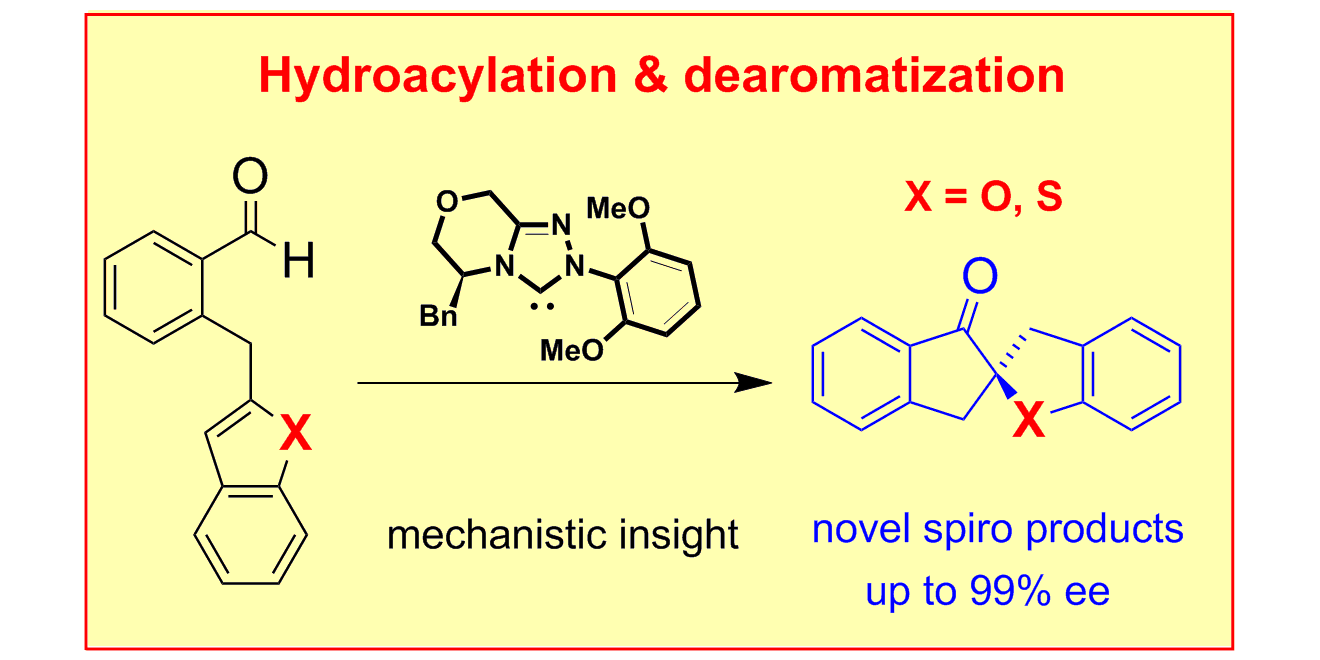
D. Janssen-Müller, M. Fleige, D. Schlüns, M. Wollenburg, C. G. Daniliuc, J. Neugebauer,* F. Glorius,*
NHC-Catalyzed Enantioselective Dearomatizing Hydroacylation of Benzofurans and Benzothiophenes for the Synthesis of Spirocycles,
ACS Catal. 2016, 6, 5735-5739.
C. Guo, M. Fleige, D. Janssen-Müller, C. G. Daniliuc, F. Glorius,
Cooperative N-Heterocyclic Carbene/Palladium-Catalyzed Enantioselective Umpolung Annulations,
J. Am. Chem. Soc. 2016, 138, 7840-7843.
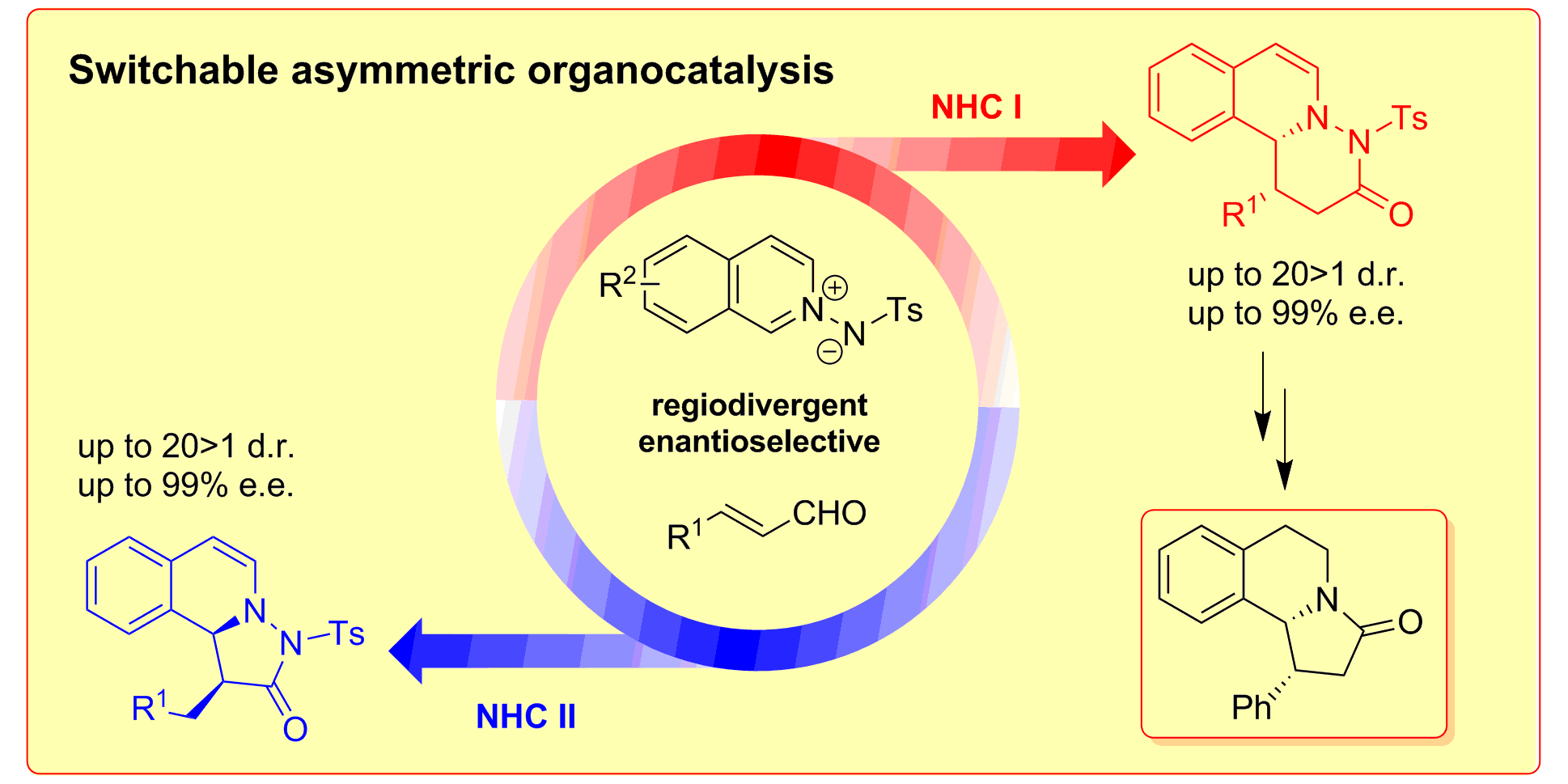
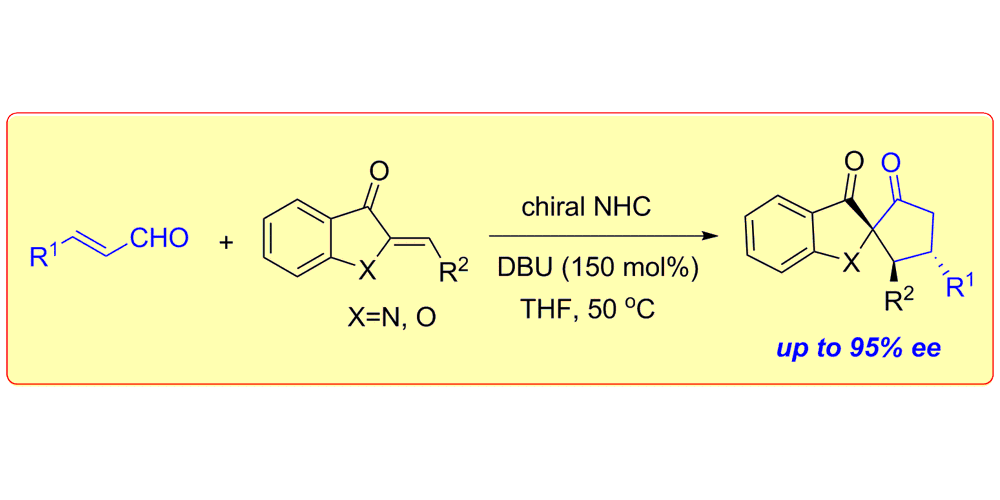
C. Guo, M. Fleige, D. Janssen-Müller, C. G. Daniliuc, F. Glorius,
Switchable selectivity in an NHC-catalysed dearomatizing annulation reaction,
Nature Chem. 2015, 7, 842-847.
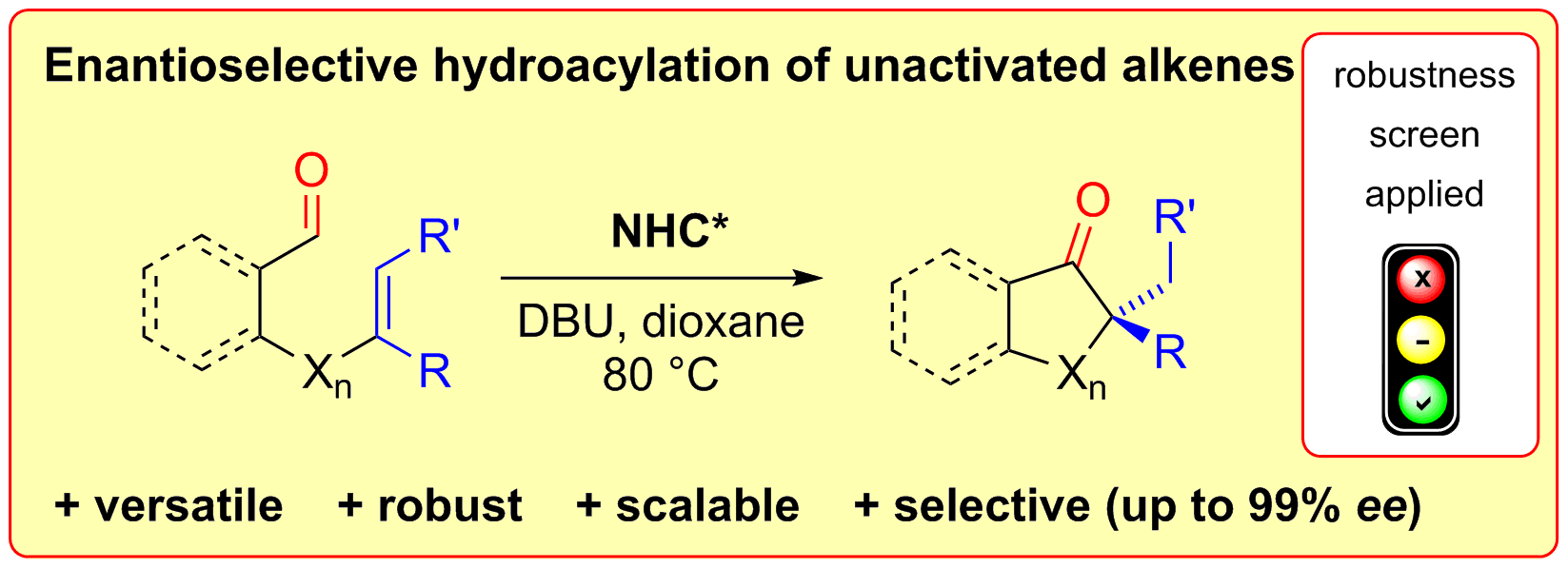
D. Janssen-Müller, M. Schedler, M. Fleige, C. G. Daniliuc, F. Glorius,
Enantioselective Intramolecular Hydroacylation of Unactivated Alkenes: An NHC-Catalyzed Robust and Versatile Formation of Cyclic Chiral Ketones,
Angew. Chem. Int. Ed. 2015, 54, 12492-12496; Angew. Chem. 2015, 127, 12671-12675.
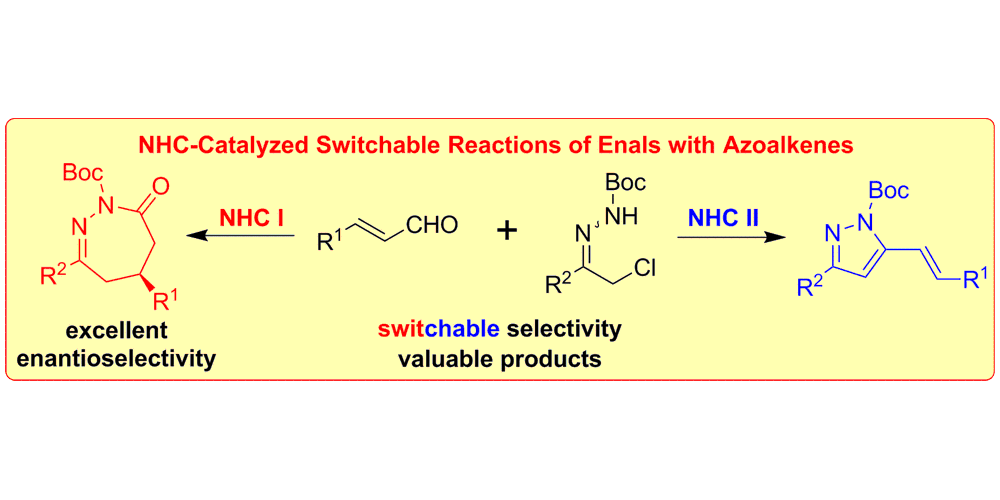
C. Guo, B. Sahoo, C. G. Daniliuc, F. Glorius,
N-Heterocyclic Carbene Catalyzed Switchable Reactions of Enals with Azoalkenes: Formal [4+3] and [4+1] Annulations for the Synthesis of 1,2-Diazepines and Pyrazoles,
J. Am. Chem. Soc. 2014, 136, 17402-17405.
J.-L. Li, B. Sahoo, C.-G. Daniliuc, F. Glorius,
Conjugate Umpolung of β,β-Disubstituted Enals by Dual Catalysis with an N-Heterocyclic Carbene and a Brønsted Acid: Facile Construction of Contiguous Quaternary Stereocenters,
Angew. Chem. Int. Ed. 2014, 53, 10515-10519; Angew. Chem. 2014, 126, 10683-10687.
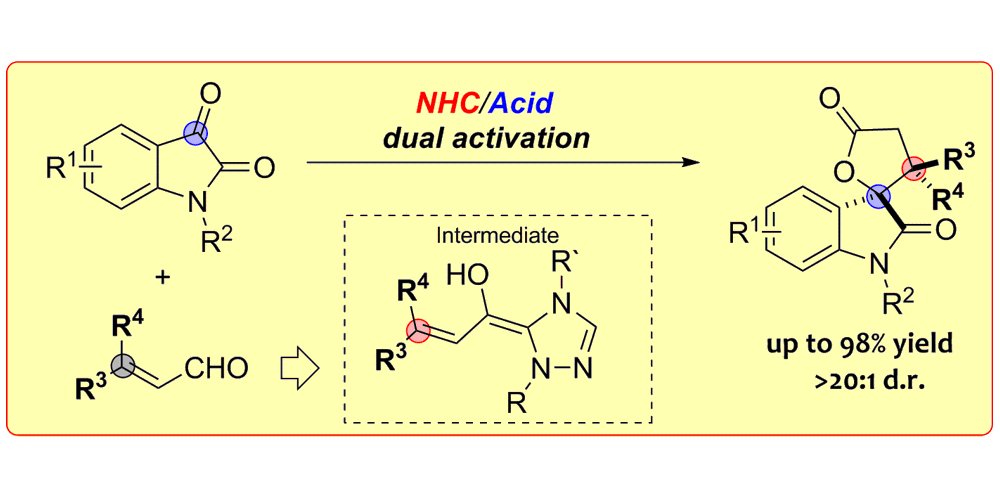
C. Guo, M. Schedler, C. G. Daniliuc, F. Glorius,
N-Heterocyclic Carbene Catalyzed Formal [3+2] Annulation Reaction of Enals: An Efficient Enantioselective Access to Spiro-Heterocycles,
Angew. Chem. Int. Ed. 2014, 53, 10232-10236; Angew. Chem. 2014, 126, 10397-10401.
Highlighted in Synfacts 2014, 10, 1207.
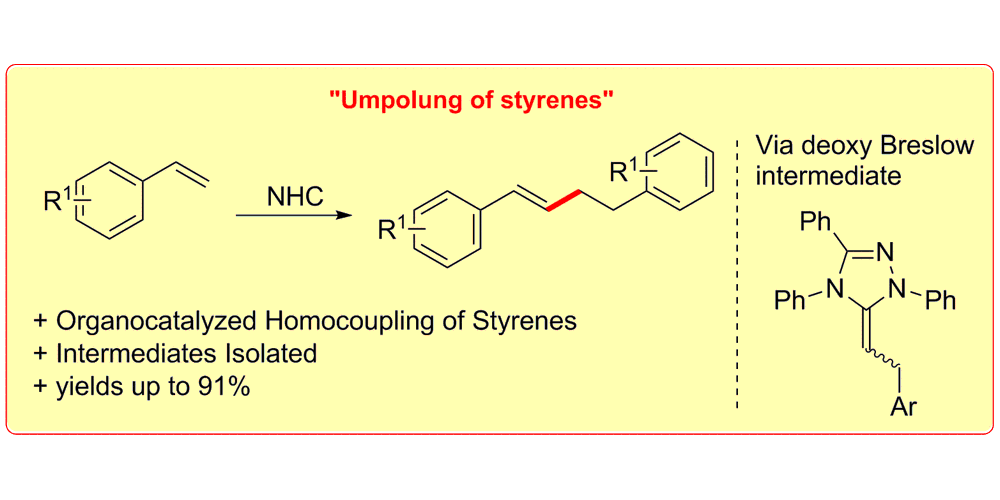
M. Schedler, N. E. Wurz, C. G. Daniliuc, F. Glorius,
N-Heterocyclic Carbene Catalyzed Umpolung of Styrenes: Mechanistic Elucidation and Selective Tail-to-Tail Dimerization,
Org. Lett. 2014, 16, 3134-3137.
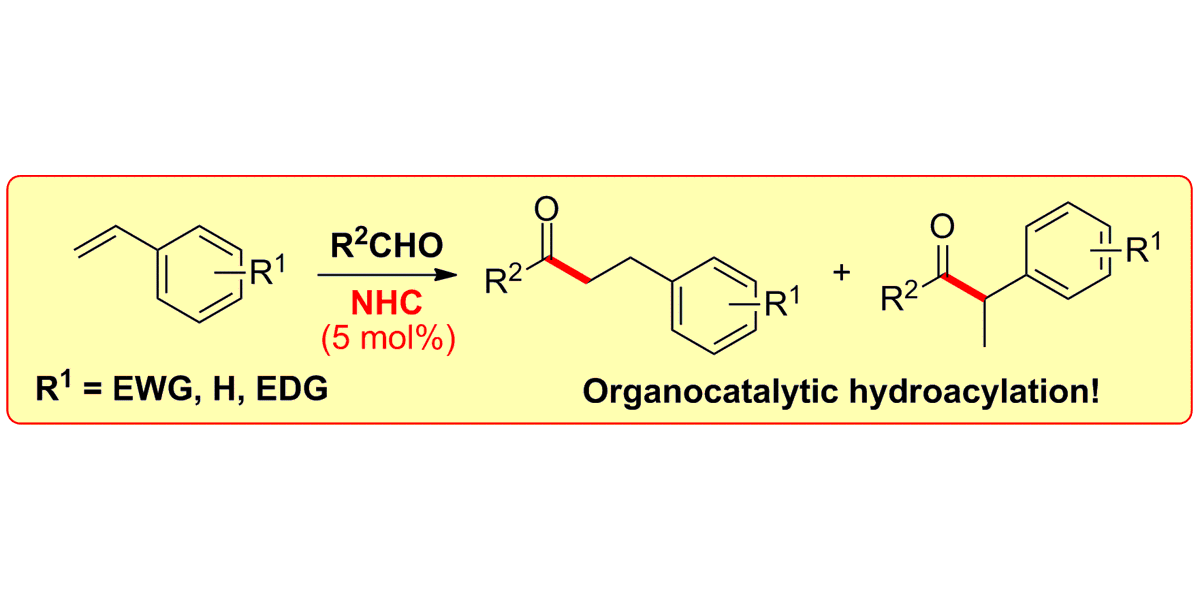
M. Schedler, D.-S. Wang, F. Glorius,
NHC-Catalyzed Hydroacylation of Styrenes,
Angew. Chem. Int. Ed. 2013, 52, 2585-2589; Angew. Chem. 2013, 125, 2645-2649.