Data Science

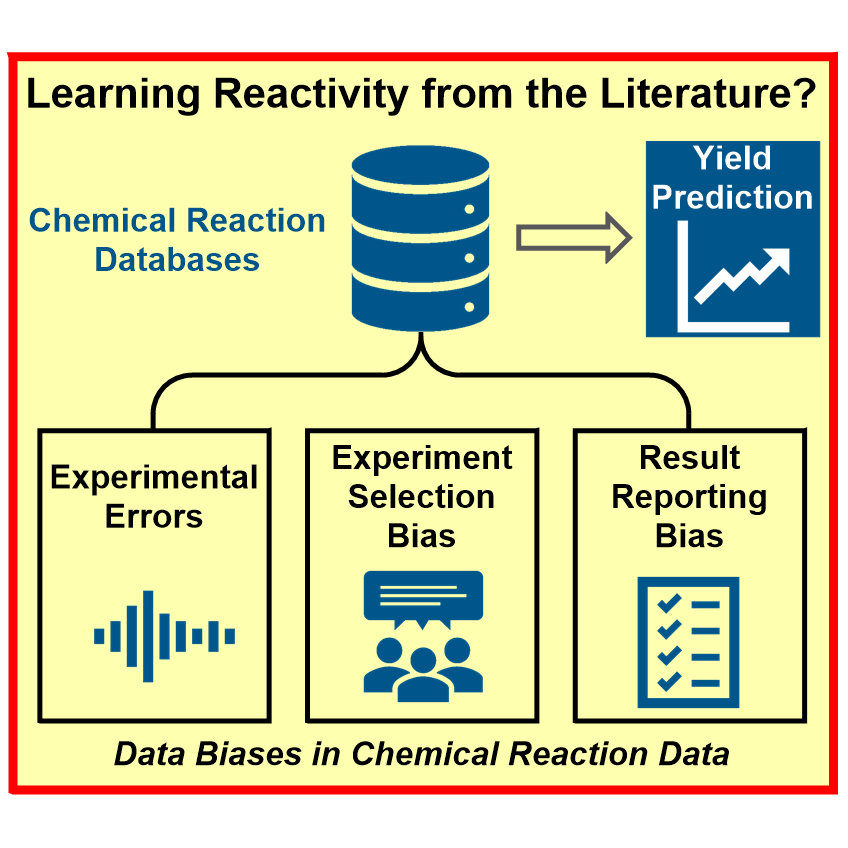
A major obstacle regarding the digitalization of organic synthesis is the availability of reaction-based data. To meet this challenge, we develop smart screening technologies for the efficient generation of high-quality data. By leveraging advanced machine learning approaches we extract valuable insights from this data and apply them to solve chemical challenges:
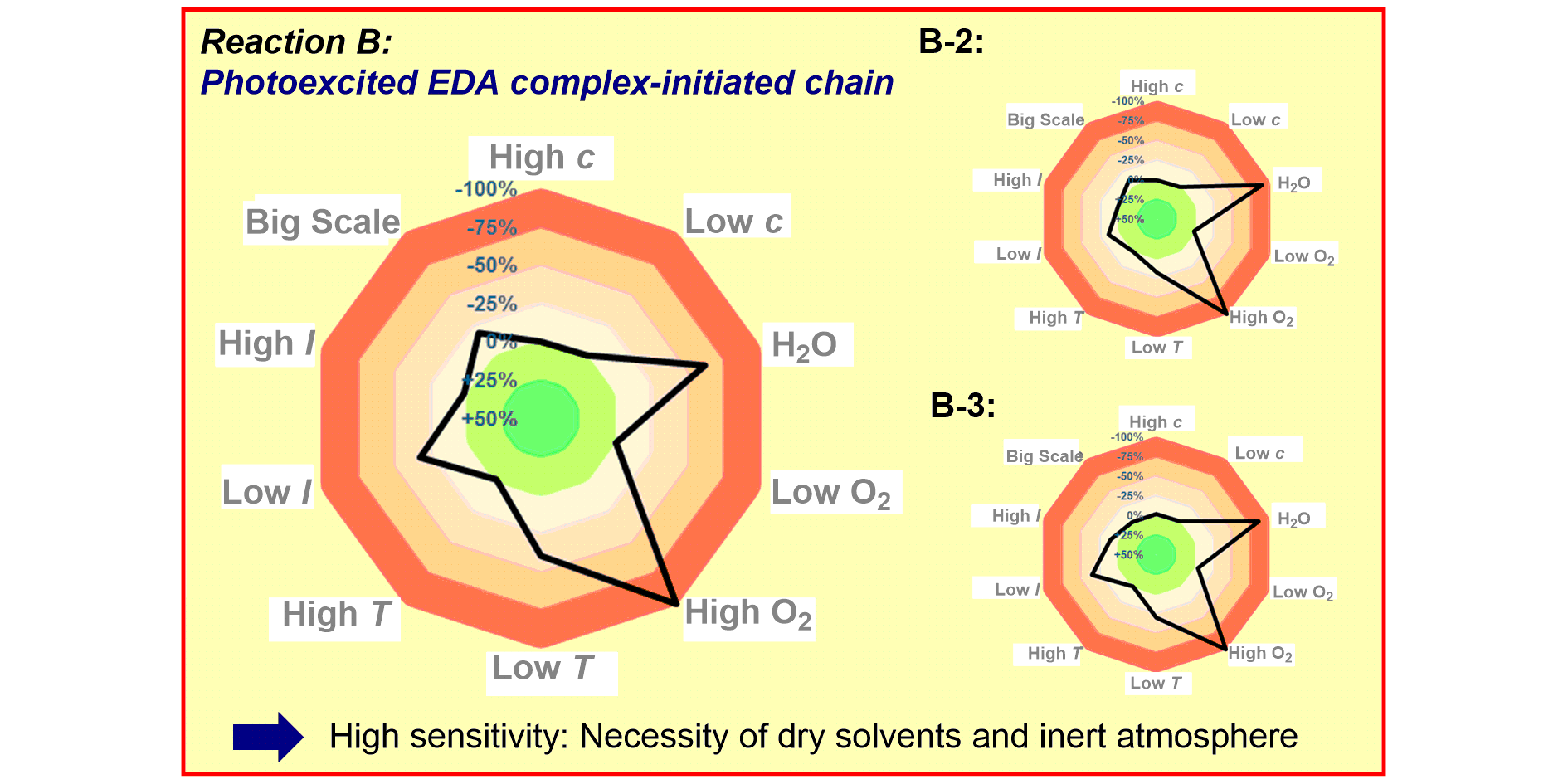
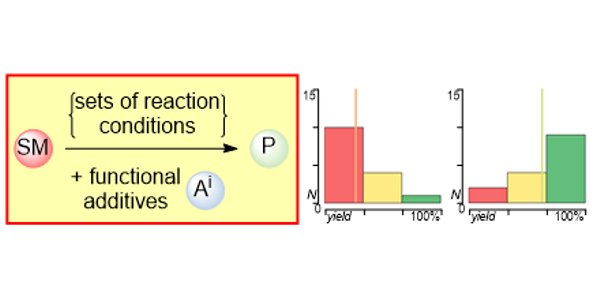
a) Reaction Assessment: For the rapid assessment of chemical reactions, we developed an additive-based robustness screen[1] to evaluate functional group tolerance and a condition-based sensitivity screen[2] to reduce reproducibility issues. Furthermore, to increase the informativeness in a reaction’s scope, we developed a data-driven substrate selection strategy.[3]
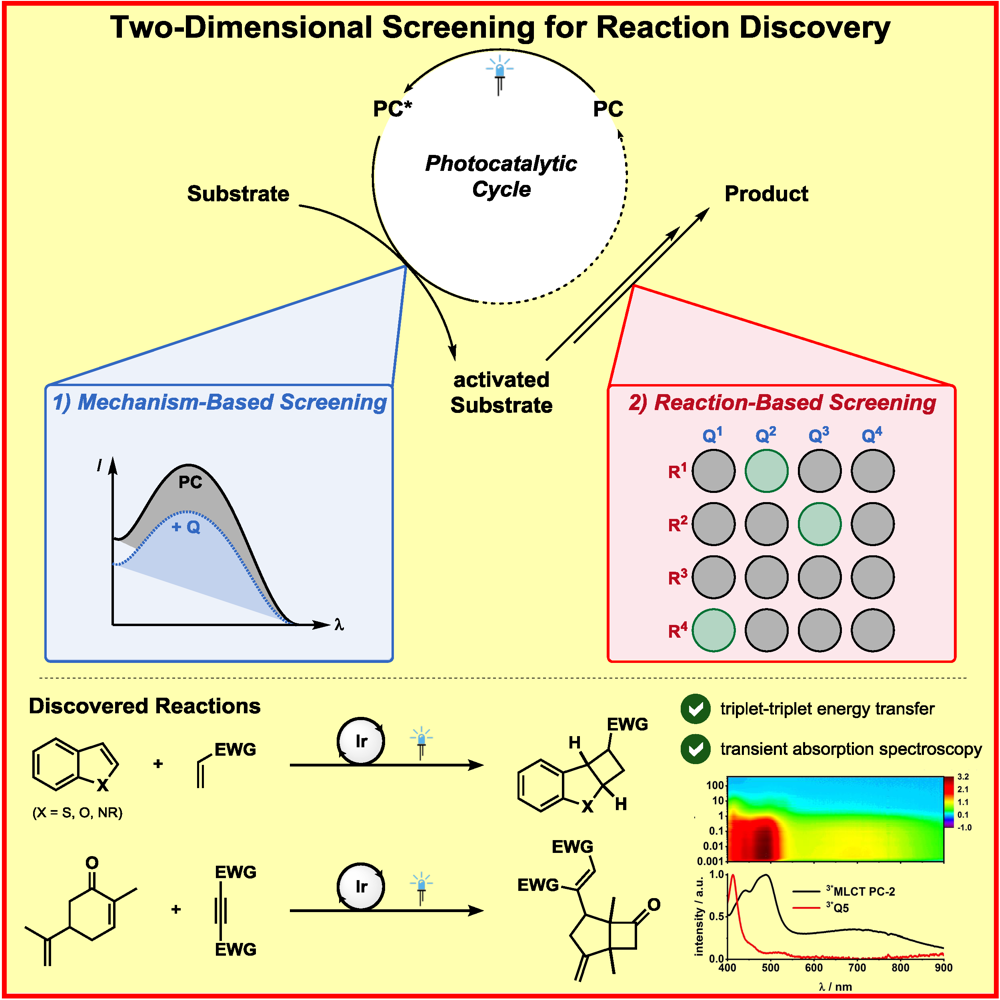
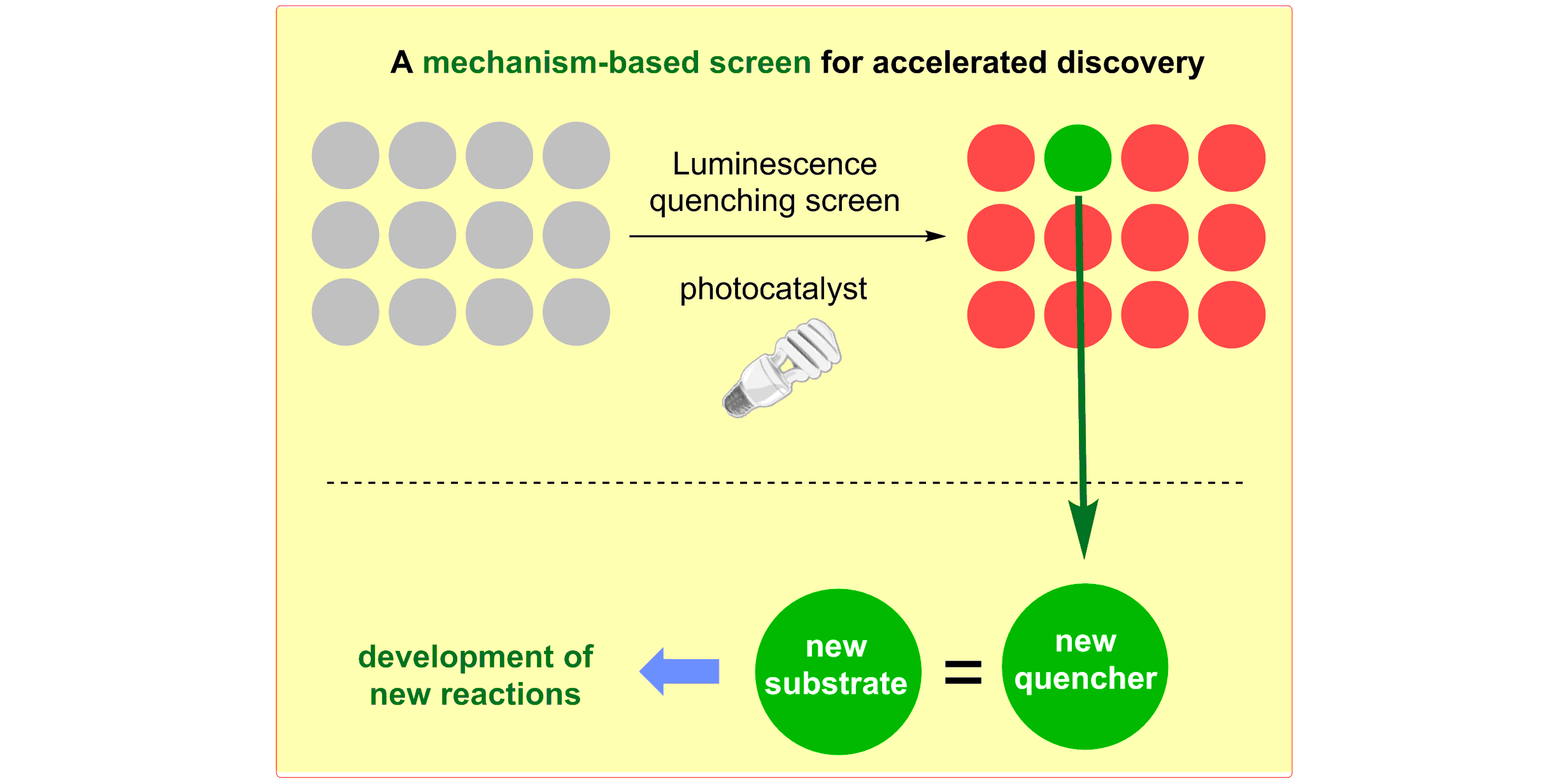
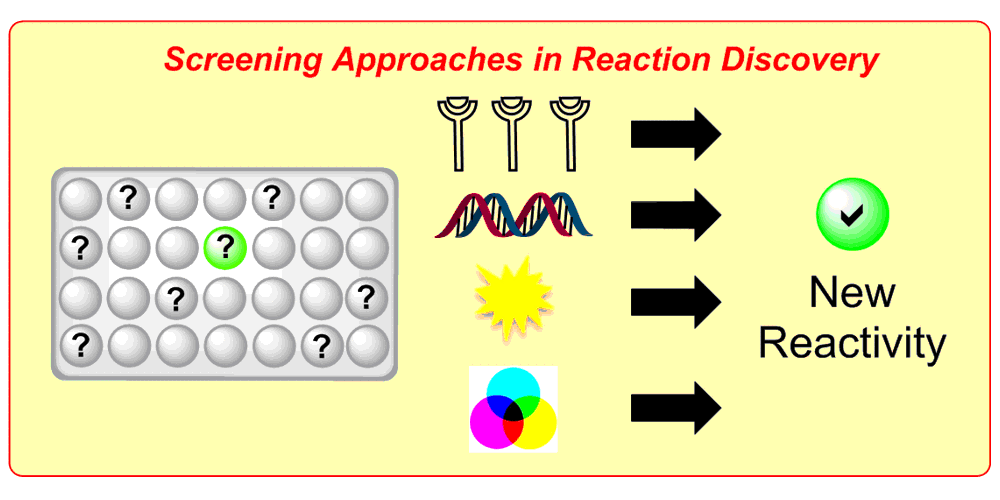
b) Accelerated Discovery: A mechanism-based screening strategy based on luminescence quenching[4] allowed for the discovery of various photochemical transformations. The serendipitous findings could further be enhanced via a two-dimensional screening approach.[5]
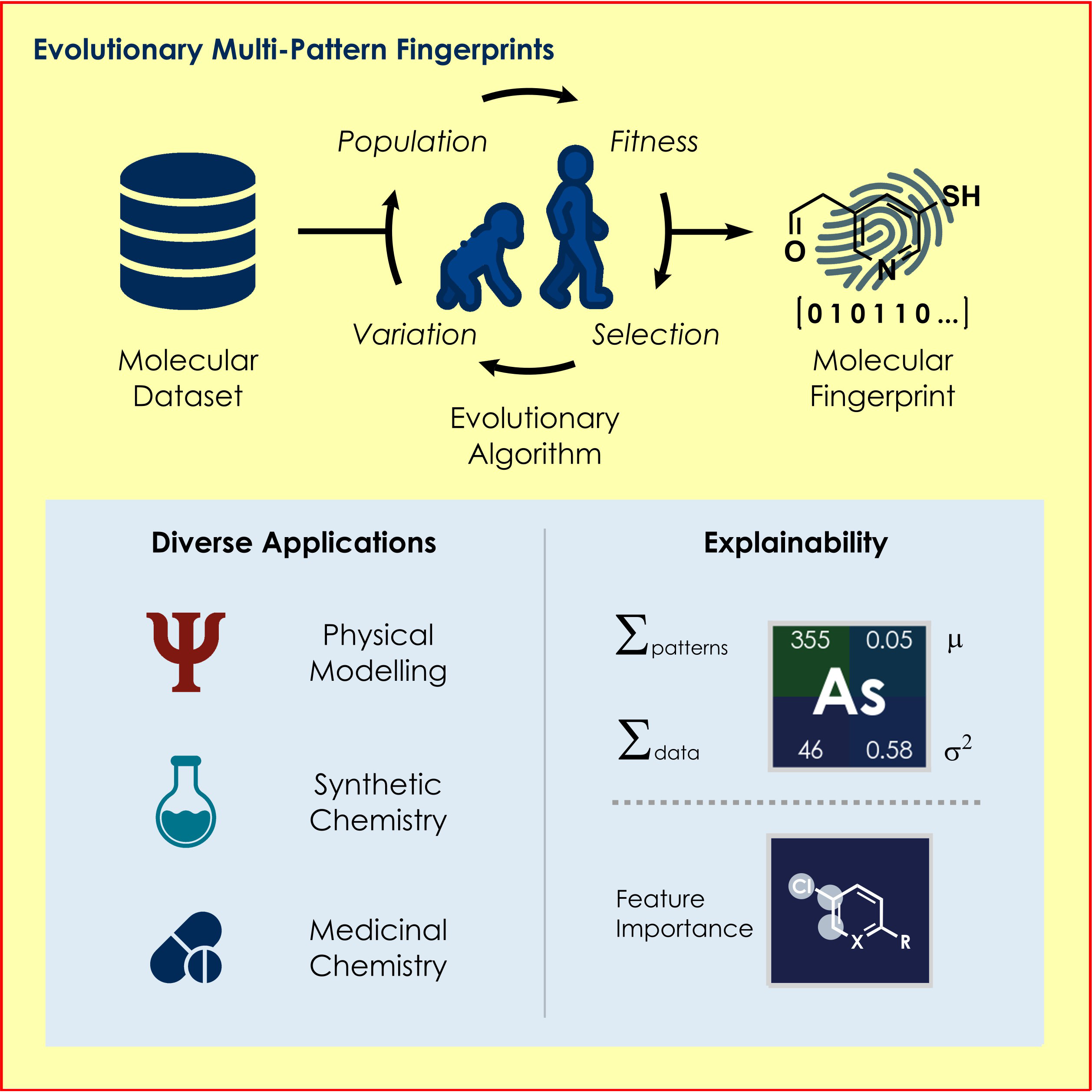
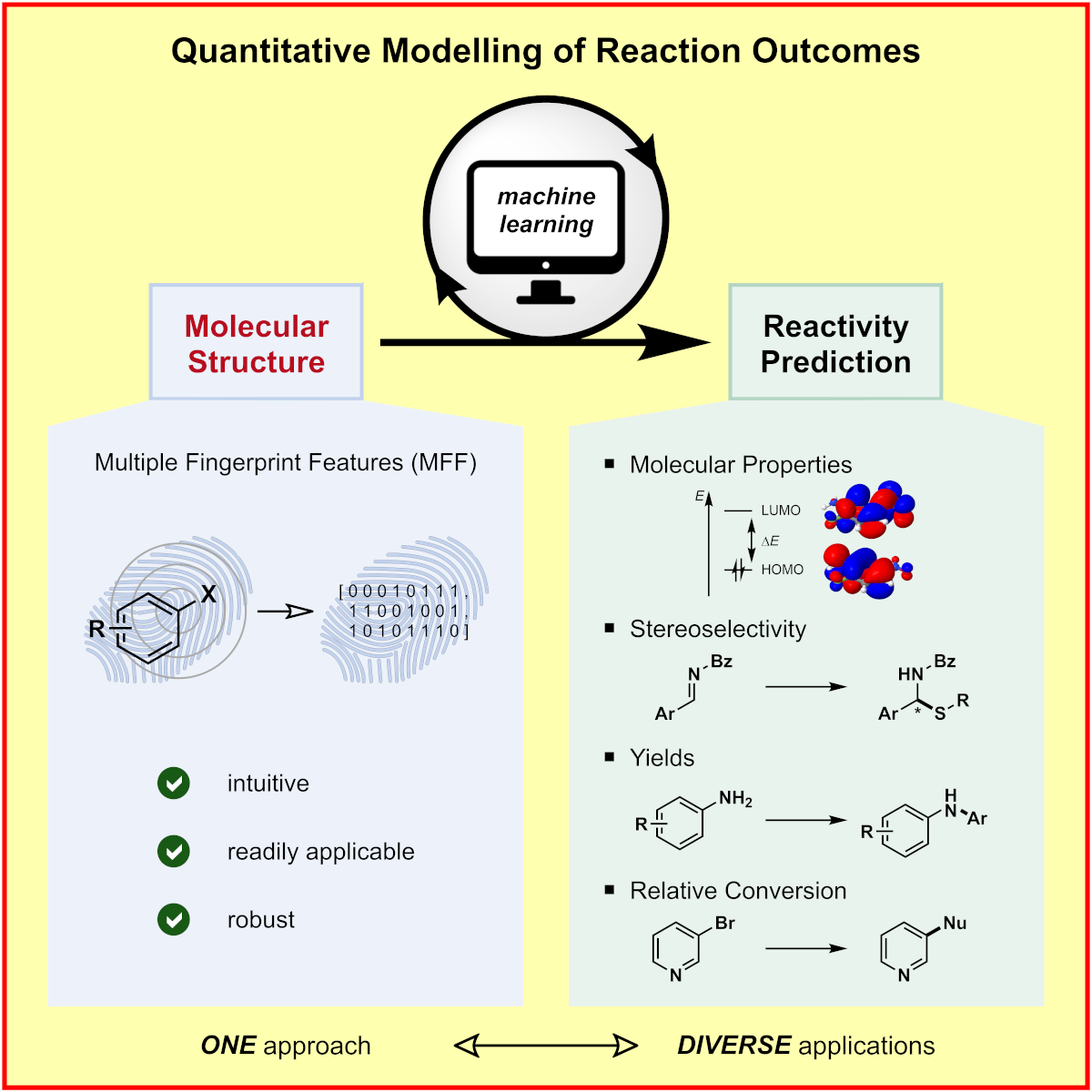
c) Molecular Machine Learning: We developed a genetic algorithm yielding problem-specific and interpretable molecular representations[6] which can be used for quantitative prediction of reactivity.[7] Moreover, we utilize high-throughput virtual screening for accelerated reaction discovery.[8]
[1] K. D. Collins, F. Glorius, Nature Chem. 2013, 5, 597. [2] L. Pitzer, F. Schäfers, F. Glorius, Angew. Chem. Int. Ed. 2019, 58, 8572. [3] D. Rana, P. M. Pflüger, N. P. Hölter, G. Tan, F. Glorius, ACS Cent. Sci. 2024, 10, 899. [4] M. N. Hopkinson, A. Gómez‐Suárez, M. Teders, B. Sahoo, F. Glorius, Angew. Chem. Int. Ed. 2016, 55, 4361. [5] F. Strieth-Kalthoff, C. Henkel, M. Teders, A. Kahnt, W. Knolle, A. Gómez-Suárez, K. Dirian, W. Alex, K. Bergander, C. G. Daniliuc, B. Abel, D. M. Guldi, F. Glorius, Chem 2019, 5, 339. [6] P. M. Pflüger, M. Kühnemund, F. Katzenburg, H. Kuchen, F. Glorius, Chem 2024, 10, 1391. [7] F. Sandfort, F. Strieth-Kalthoff, M. Kühnemund, C. Beecks, F. Glorius, Chem 2020, 6, 1379. [8] L. Schlosser, D. Rana, P. M. Pflüger, F. Katzenburg, F. Glorius, J. Am. Chem. Soc. 2024, 146, 13266.
Please click on the graphical abstracts to come to the original publication
S. P. Schmid,§ L. Schlosser,§ F. Glorius,* K. Jorner,*
Catalysing (organo-) catalysis: Trends in the application of machine learning to enantioselective organocatalysis,
Beilstein J. Org. Chem. 2024, 20, 2280-2304.
§ These authors contributed equally.
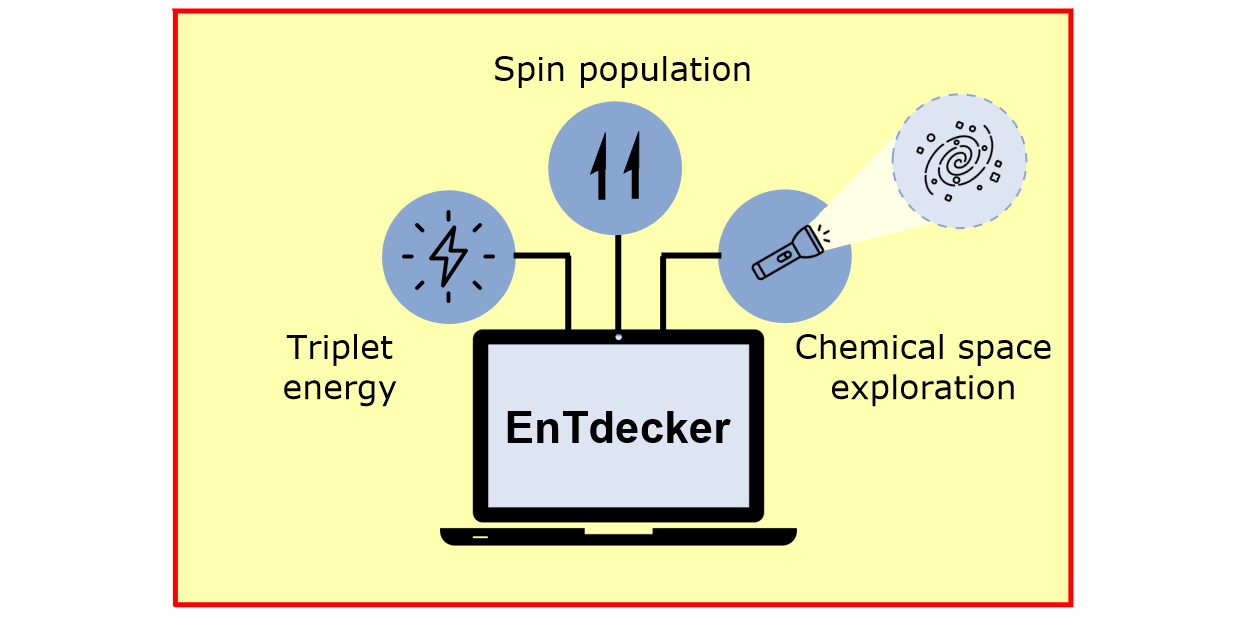
L. Schlosser, D. Rana, P. Pflüger, F. Katzenburg, F. Glorius,*
EnTdecker – A machine learning-based platform for guiding substrate discovery in energy transfer catalysis,
J. Am. Chem. Soc. 2024, 146, 13266-13275.
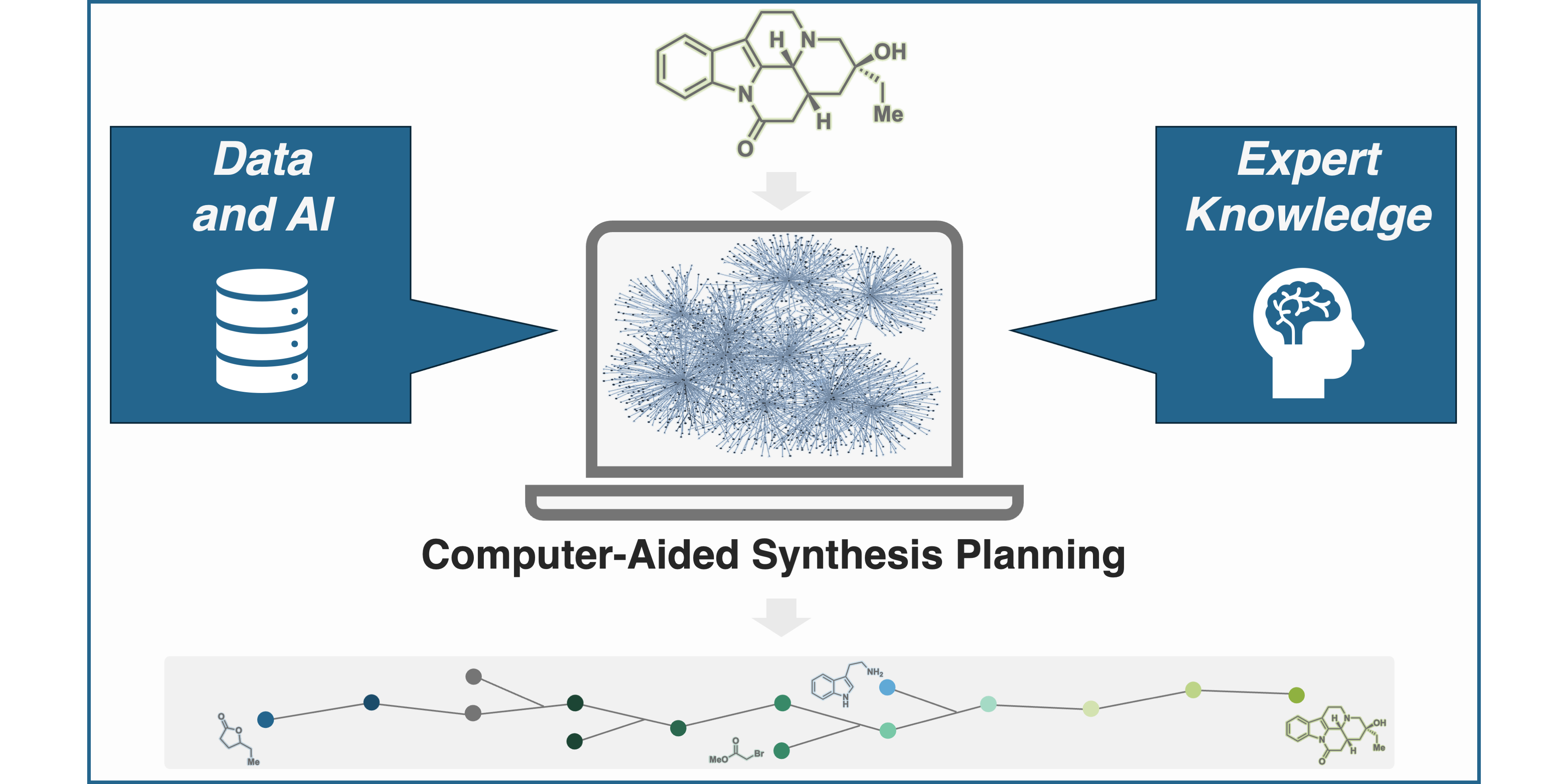
F. Strieth-Kalthoff,§ S. Szymkuc,§ K. Molga,§ A. Aspuru-Guzik, F. Glorius,* B. A. Grzybowski,*
Artificial Intelligence for Retrosynthetic Planning Needs Both Data and Expert Knowledge,
J. Am. Chem. Soc. 2024, 146, 11005-11017.
§ These authors contributed equally.
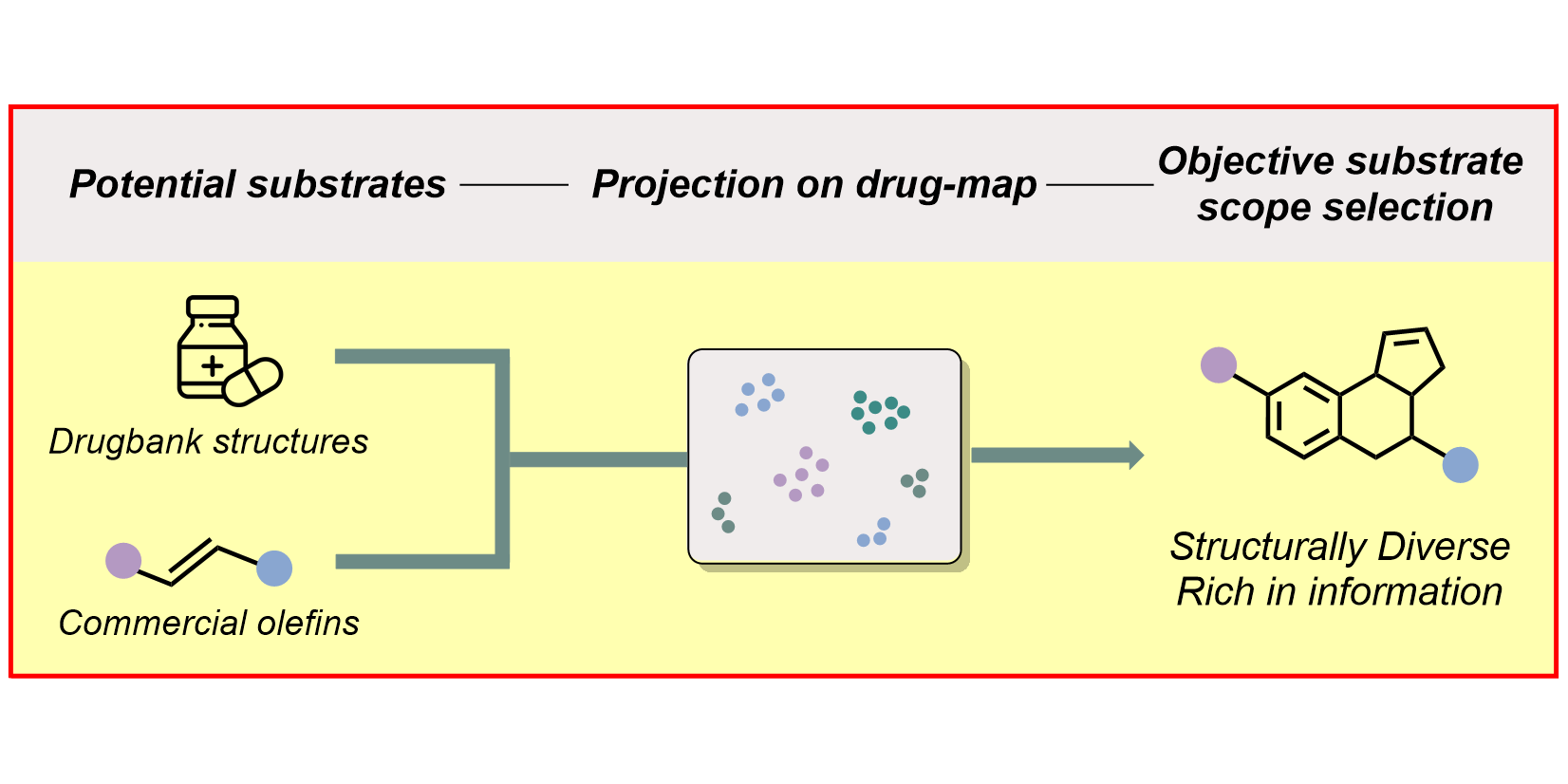
D. Rana, P. M. Pflüger, N. P. Hölter, G. Tan, F. Glorius,
Standardizing Substrate Selection: A Strategy toward Unbiased Evaluation of Reaction Generality,
ACS Cent. Sci. 2024, 10, 899-906.
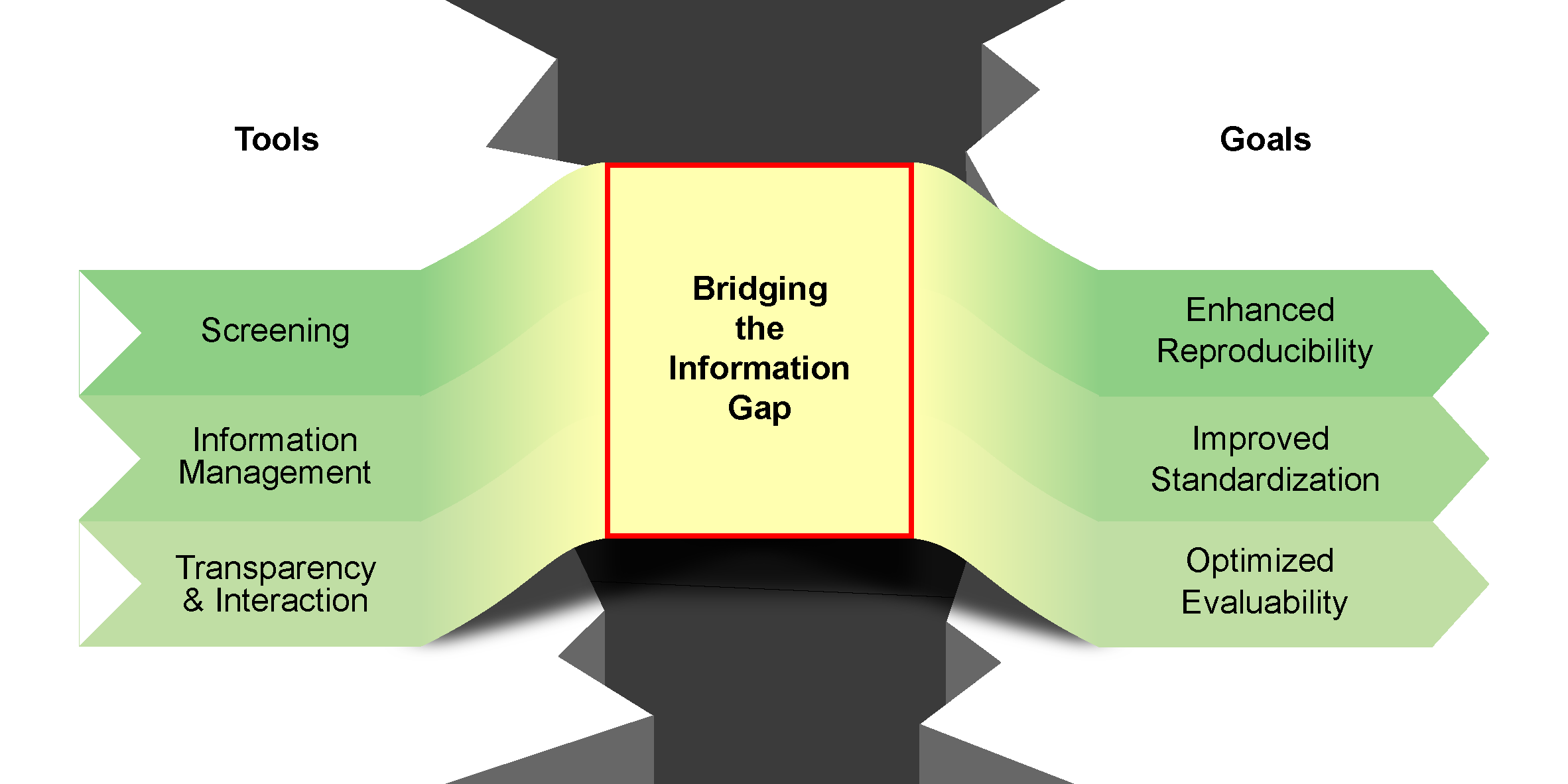
M. L. Schrader,§ F. R. Schäfer,§ F. Schäfers,§ F. Glorius,
Bridging the information gap in organic chemical reactions,
Nat. Chem. 2024, 16, 491-498.
§ These authors contributed equally.
P. M. Pflüger,§ M. Kühnemund,§ F. Katzenburg,§ H. Kuchen, F. Glorius,
An evolutionary algorithm for interpretable molecular representations,
Chem 2024, 10, 1391-1405.
§ These authors contributed equally; selected for cover image.
F. Strieth-Kalthoff,§ F. Sandfort,§ M. Kühnemund, F. R. Schäfer, H. Kuchen, F. Glorius,
Machine Learning for Chemical Reactivity: The Importance of Failed Experiments,
Angew. Chem. Int. Ed. 2022, 61 (29), e202204647; Angew. Chem. 2022, 134 (29), e202204647.
P. M. Pflüger, F. Glorius,
Molecular Machine Learning: The Future of Synthetic Chemistry?,
Angew. Chem. Int. Ed. 2020, 59, 18860-18865; Angew. Chem. 2020, 132, 19020-19025.
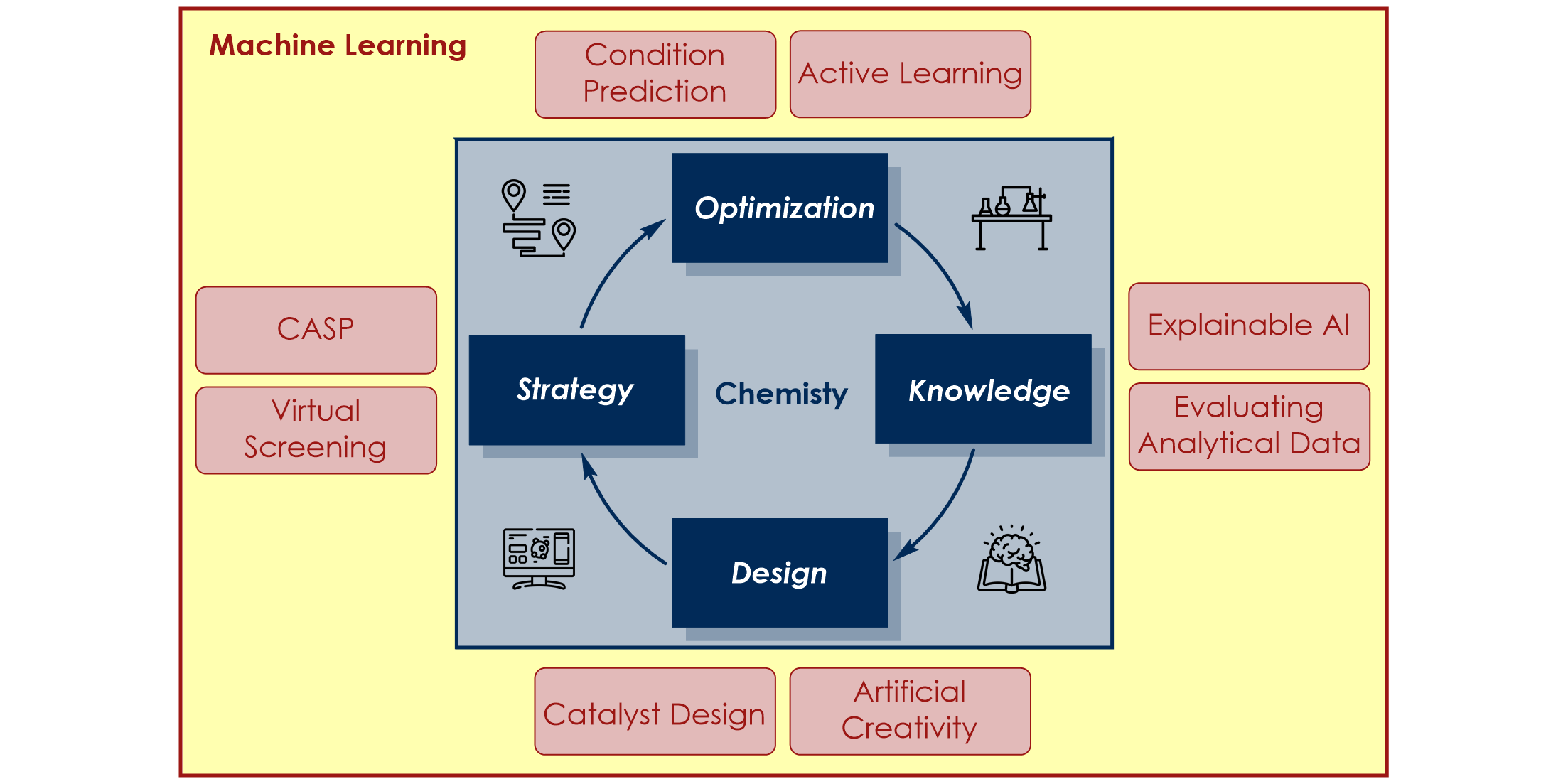
F. Strieth-Kalthoff, F. Sandfort, M. H. S. Segler, F. Glorius,
Machine Learning the Ropes: Principles, Applications and Directions in Synthetic Chemistry,
Chem. Soc. Rev. 2020, 49, 6154-6168.
F. Sandfort,§ F. Strieth-Kalthoff,§ M. Kühnemund,§ C. Beecks, F. Glorius,*
A Structure-Based Platform for Predicting Chemical Reactivity,
Chem 2020, 6, 1379-1390.
ChemRxiv (Preprint) 2019. DOI
§ All three authors contributed equally.
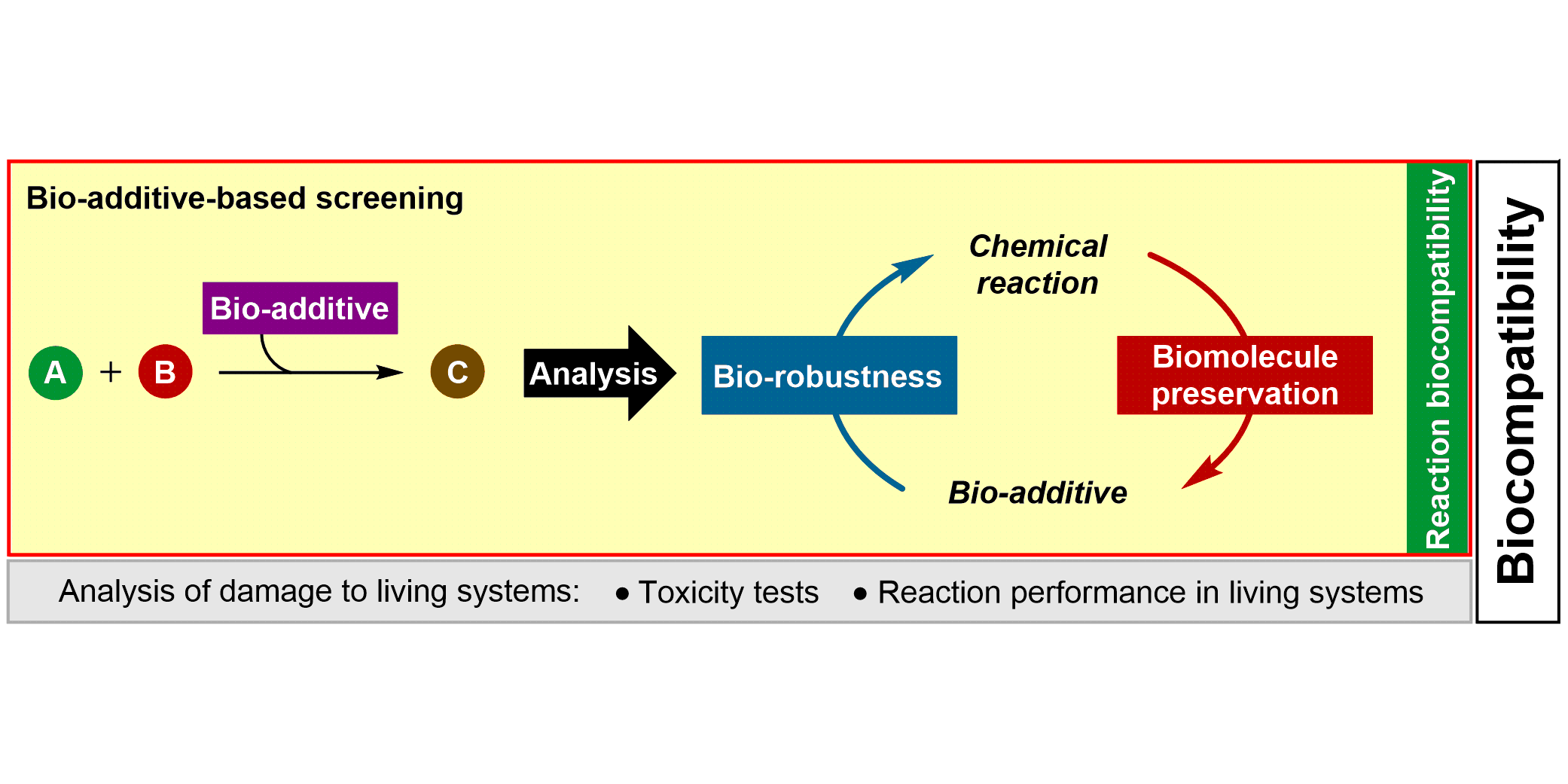
L. Anhäuser, M. Teders, A. Rentmeister,* F. Glorius,*
Bio-additive-based screening: toward evaluation of the biocompatibility of chemical reactions,
Nature Protoc. 2019, 14, 2599-2626.
F. Strieth-Kalthoff, C. Henkel, M. Teders, A. Kahnt, W. Knolle, A. Gómez-Suárez, K. Dirian, W. Alex, K. Bergander, C. G. Daniliuc, B. Abel, D. M. Guldi,* F. Glorius,*
Discovery of Unforeseen Energy-Transfer-Based Transformations Using a Combined Screening Approach,
Chem 2019, 5, 2183-2194.
L. Pitzer,§ F. Schäfers,§ F. Glorius,
Rapid Assessment of the Reaction Condition-Based Sensitivity of Chemical Transformations,
Angew. Chem. Int. Ed. 2019, 58, 8572-8576; Angew. Chem. 2019, 131, 8660-8664.
§ Both authors contributed equally.
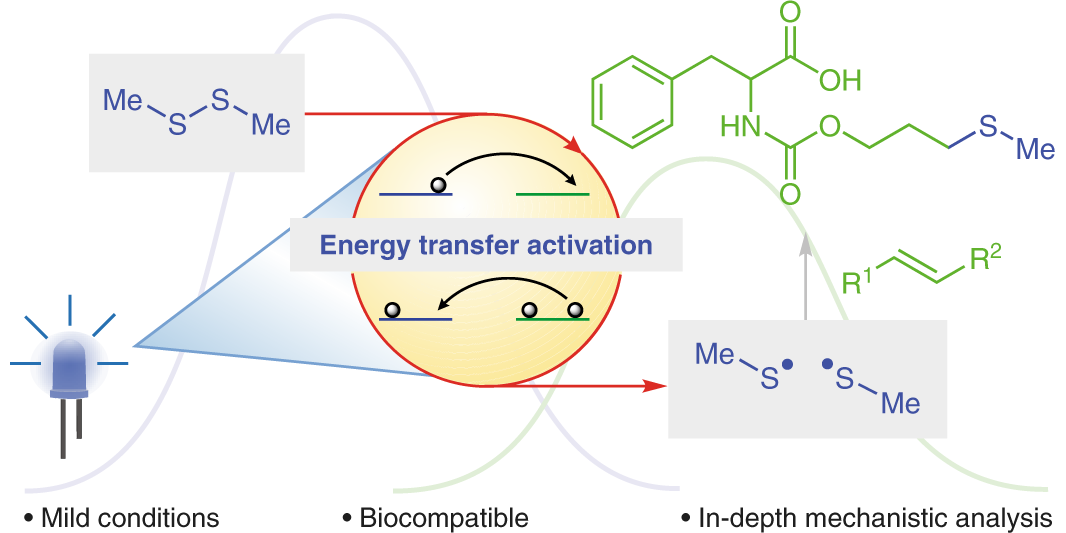
M. Teders, C. Henkel, L. Anhäuser, F. Strieth-Kalthoff, A. Goméz-Suárez, R. Kleinmans, A. Kahnt, A. Rentmeister, D. M. Guldi,* F. Glorius,*
The energy-transfer-enabled biocompatible disulfide–ene reaction,
Nat. Chem. 2018, 10, 981-988.
T. Gensch§, M. Teders§, F. Glorius,
An Approach to Comparing the Functional Group Tolerance of Reactions,
J. Org. Chem. 2017, 82, 9154-9159.
§ Both authors contributed equally.
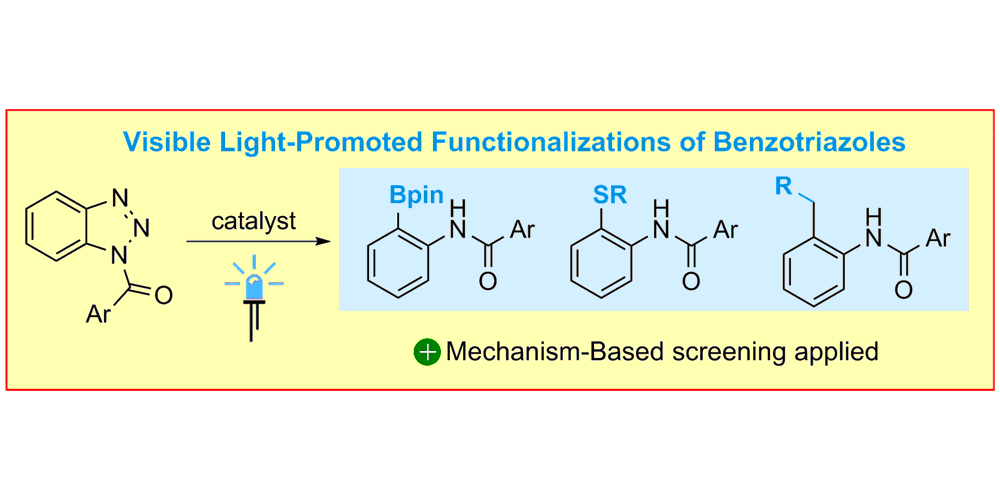
M. Teders, A. Gómez-Suárez§, L. Pitzer§, M. N. Hopkinson, F. Glorius,
Diverse Visible-Light-Promoted Functionalizations of Benzotriazoles Inspired by Mechanism-Based Luminescence Screening,
Angew. Chem. Int. Ed. 2017, 56, 902-906; Angew. Chem. 2017, 129, 921-925.
M. N. Hopkinson, A. Gomez-Suarez, M. Teders, B. Sahoo, F. Glorius,
Accelerated Discovery in Photocatalysis using a Mechanism-Based Screening Method,
Angew. Chem. Int. Ed. 2016, 55, 4361-4366; Angew. Chem. 2016, 128, 4434-4439.
K. D. Collins, T. Gensch, F. Glorius,
Contemporary screening approaches to reaction discovery and development,
Nat. Chem. 2014, 6, 859–871.
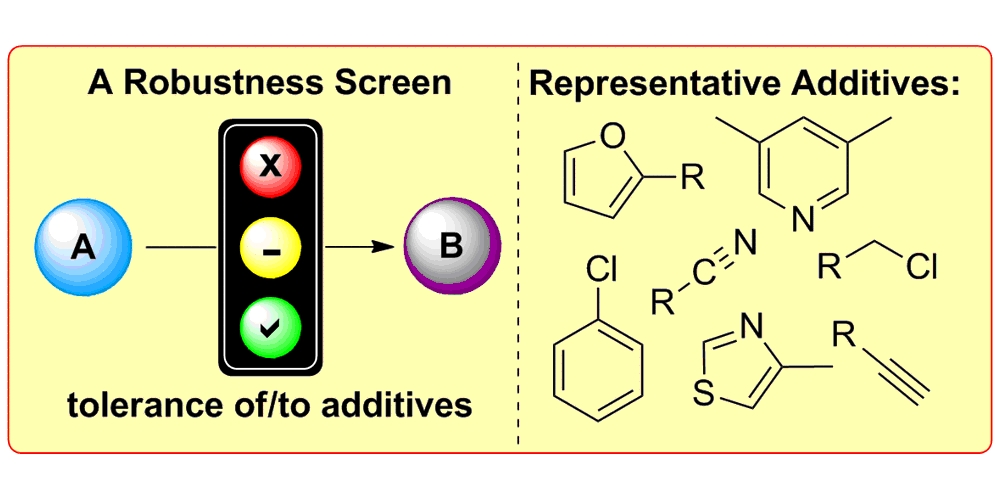
K. D. Collins, A. Rühling, F. Glorius,
Application of a Robustness Screen for the evaluation of synthetic organic methodology,
Nat. Protoc. 2014, 9, 1348-1353.
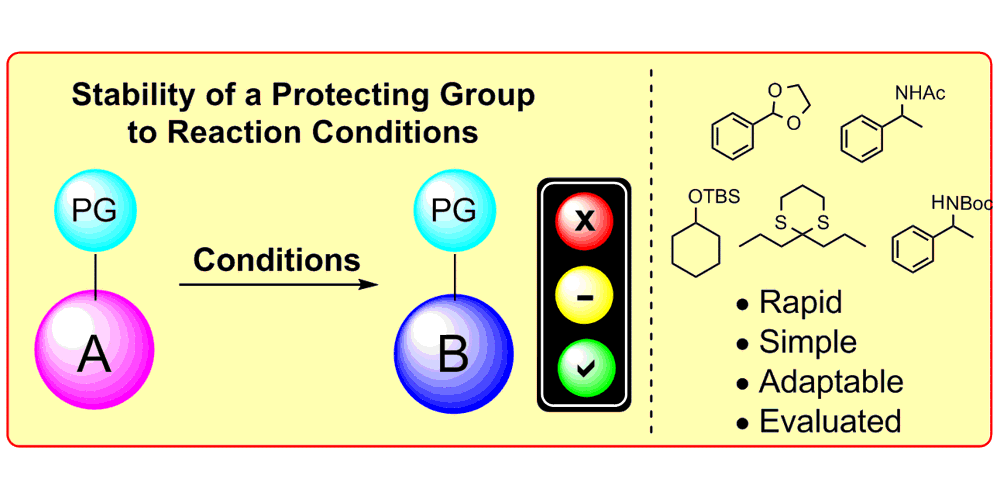
K. D. Collins,* A. Rühling, F. Lied, F. Glorius,*
Rapid Assessment of Protecting Group Stability Using a Robustness Screen,
Chem. Eur. J. 2014, 20, 3800-3805.
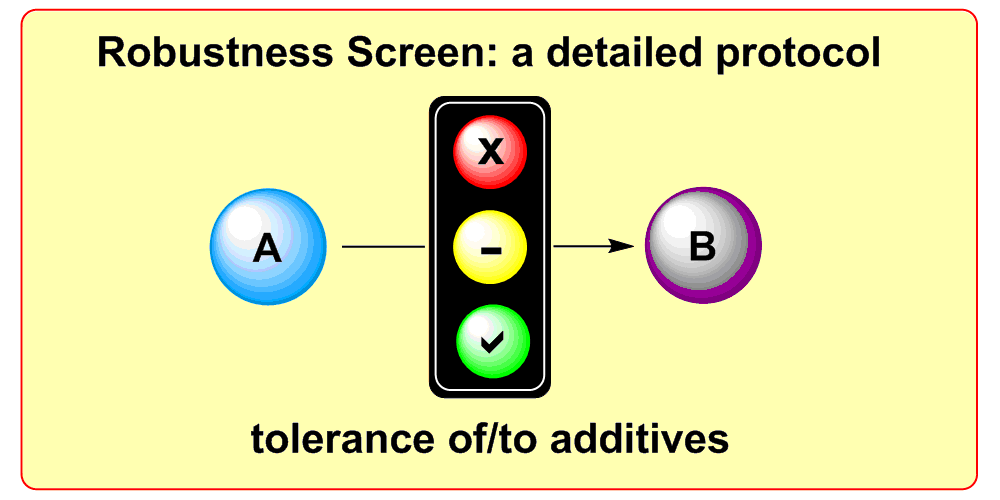
K. D. Collins, F. Glorius,
A Robustness Screen for the Rapid Assessment of Chemical Reactions,
Nature Chem. 2013, 5, 597-601.
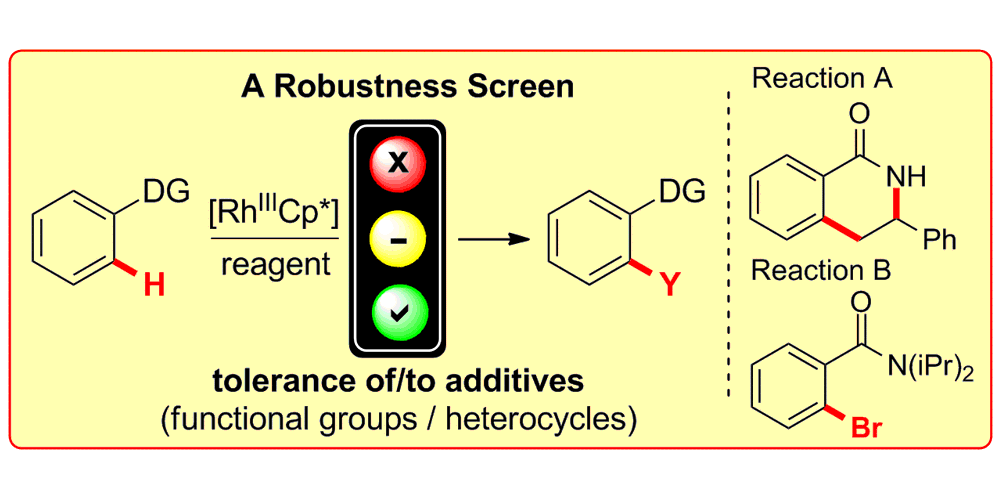
K. D. Collins, F. Glorius,
Employing a Robustness Screen: Rapid Assessment of Rhodium(III)-catalysed C-H Activation Reactions,
Tetrahedron 2013, 69, 7817-7825.