2025
151. Enantioselective Energy Transfer-Enabled Cyclization using a Privileged Al-Salen Photocatalyst
J. Soika, C. Onneken, T. Wiegmann, T. Morack, L. Lindfeld, M. Hebenbrock, C. Mück-Lichtenfeld, J. Neugebauer and R. Gilmour, ChemRxiv 2024, doi:10.26434/chemrxiv-2024-9g3qz.
© Ryan Gilmour
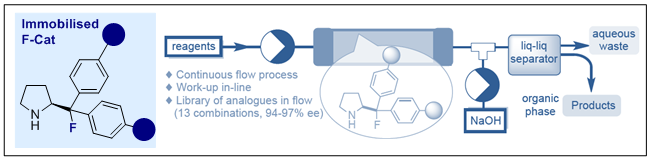
150. Design, Synthesis and Pre-Clinical Evaluation of a Carbohydrate Tracer for 18F-PET Multiple Sclerosis Imaging
K. Siebold, L. Göbel, C. P. Konken, A. Faust and R. Gilmour, Eur. J. Org. Chem. 2025, accepted for publication.
Invited contribution for the special edition of the European Journal of Organic Chemistry dedicated to fluorine chemistry and its applications.
© Ryan Gilmour
2024
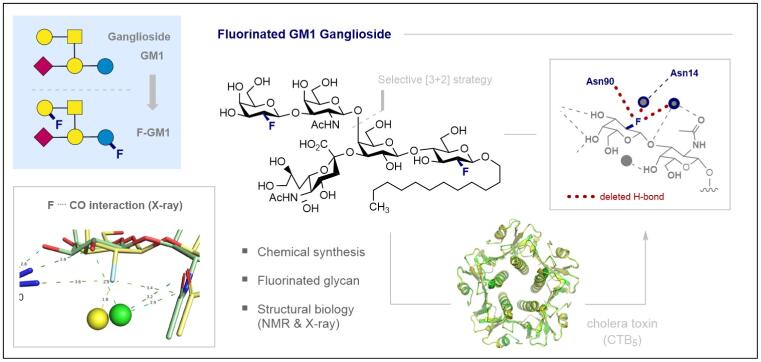
149. Probing the Origin of Affinity in the GM1-Cholera Toxin Complex through Site-selective Editing with Fluorine
C. Jordan§, T. Hayashi§, A. Löbbert, J. Fan, C. S. Teschers, K. Siebold, M. Aufiero, F. Pape, E. Campbell, A. Axer, K. Bussmann, K. Bergander, J. Köhnke, A. D. Gossert and R. Gilmour, ACS Cent. Sci. 2024, 10, 1481–1489.Highlighted in ACS Central Science: https://pubs.acs.org/doi/10.1021/acscentsci.4c01206
© Ryan Gilmour 148. Regioselective Fluorination of Allenes Enabled by I(I)/I(III) Catalysis
Z.-X. Wang, Y. Xu and R. Gilmour, Nature Communications 2024, 15, 5770.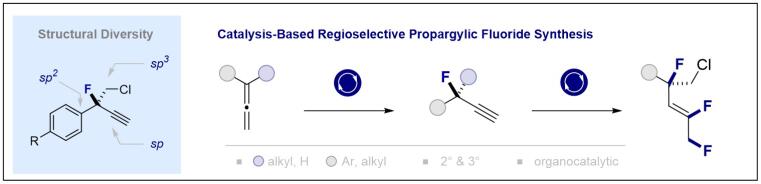
© Ryan Gilmour 147. A Fluorinated Sialic Acid Vaccine Lead Against Meningitis B and C
C. Jordan§, K. Siebold§, P. Priegue§, P. H. Seeberger and R. Gilmour, J. Am. Chem. Soc. 2024, 146, 15366–15375.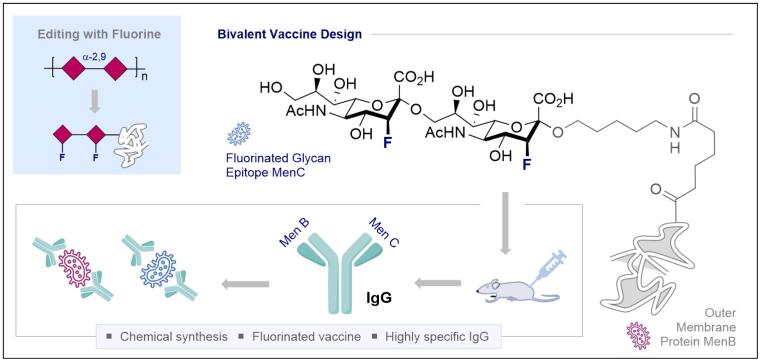
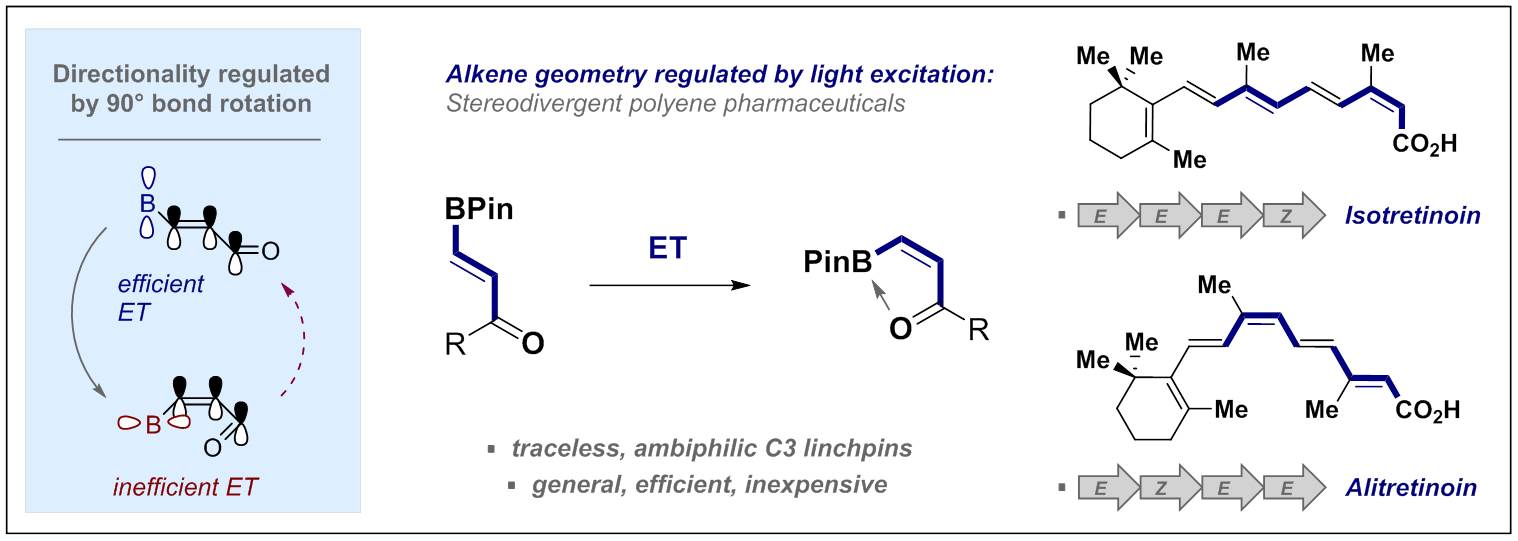
© Ryan Gilmour 146. Regio- and Stereo-Selective Isomerization of Borylated 1,3-Dienes Enabled by Selective Energy Transfer Catalysis
B. Kweon, L. Blank, J. Soika, A. Messara, C. G. Daniliuc and R. Gilmour, Angew. Chem. Int. Ed. 2024, e202404233.
© Ryan Gilmour 145. Catalytic Ring Expanding Difluorination: An Enantioselective Platform to Access β,β-Difluorinated Carbocycles
L. Ruyet, C. Roblick, J. Häfliger, Z.-X. Wang, T. J. Stoffels, C. G. Daniliuc and R. Gilmour, Angew. Chem. Int. Ed. 2024, e202403957.
© Ryan Gilmour
2023
144. Para-Selective Dearomatization of Phenols by I(I)/I(III) Catalysis-Based Fluorination
T. Stünkel, K. Siebold, D. Okumatsu, K. Murata, L. Ruyet, C. G. Daniliuc and R. Gilmour, Chem. Sci. 2023, 14, 13574 - 13580.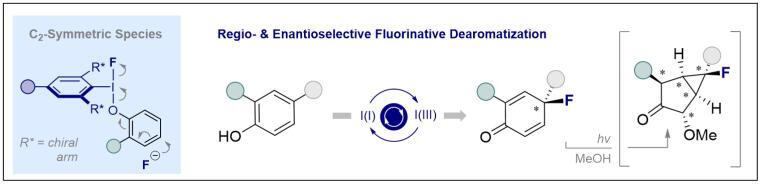
© Ryan Gilmour
143. Regioselective, catalytic 1,1-difluorination of enynes
Z.-X. Wang, K. Livingstone, C. Hümpel, C. G. Daniliuc, C. Mück-Lichtenfeld and R. Gilmour, Nature Chemistry 2023, 10.1038/s41557-023-01344-5.Highlighted by the University of Münster: https://www.uni-muenster.de/news/view.php?cmdid=13638
Highlighted by myScience:https://www.myscience.org/en/news/2023/chemists_present_method_for_the_fluorination_of_enines-2023-uni-muenster
Highlighted by Chem Europe:https://www.chemeurope.com/en/news/1181839/chemists-present-method-for-the-fluorination-of-enines.html
Highlighted by NewsBeezer:https://newsbeezer.com/india/chemists-present-method-for-fluorination-of-enynes/
Highlighted by Bionity.com:https://www.bionity.com/en/news/1181839/chemists-present-method-for-the-fluorination-of-enines.html?WT.mc_id=ca0068
Highlighted in Nature Chemistry:https://www.nature.com/articles/s41557-023-01352-5
Highlighted in Synfacts: https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0043-1763833
142. Light Enabled Deracemization of Cyclopropanes by Al Salen Photocatalysis
C. Onneken, T. Morack, J. Soika, O. Sokolova, N. Niemeyer, C. Mück-Lichtenfeld, C. G. Daniliuc, J. Neugebauer and R. Gilmour, Nature 2023, 621, 753-759.Highlighted as Synfact of the month by M. Lautens and A. Torelli: Synfacts 2023, 19, 1099. DOI: 10.1055/s-0042-1752274. https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0042-1752274?device=mobile&innerWidth=980&offsetWidth=980
Highlighted by the WWU Münster: https://www.uni-muenster.de/news/view.php?cmdid=13525
Highlighted by Chem Europe: https://www.chemeurope.com/en/news/1181399/light-regulates-structural-conversion-of-chiral-molecules.html
Highlighted by AAAS EurekAlert: https://www.eurekalert.org/news-releases/999469
Highlighted by Phys.Org: https://phys.org/news/2023-08-chemists-successfully-conversion-chiral-molecules.html
Highlighted by myScience: https://www.myscience.org/en/news/2023/light_regulates_structural_conversion_of_chiral_molecules-2023-uni-muenster
Highlighted by idw – Informationsdienst Wissenschaft: https://idw-online.de/de/news819476
Highlighted by Science Daily: https://www.sciencedaily.com/releases/2023/08/230824111848.htm
Highlighted by Analytik News: https://analytik.news/presse/2023/508.html
Highlighted by sciencenewsnet.in: https://sciencenewsnet.in/light-controls-chiral-molecule-structure/
Highlighted by Verve Times: https://vervetimes.com/structural-conversion-of-chiral-molecules-is-controlled-by-light/
Highlighted by Mirage News: https://www.miragenews.com/light-controls-structural-shift-in-chiral-1071556/
Highlighted by SwiftTelecast: https://swifttelecast.com/light-regulates-structural-conversion-of-chiral-molecules/
Highlighted by Bionity.com: https://www.bionity.com/en/news/1181399/light-regulates-structural-conversion-of-chiral-molecules.html
Highlighted by Labor Praxis: https://www.laborpraxis.vogel.de/chirale-molekuele-trennen-lichtgesteuerte-reaktion-a-4c172a4ceed27e48d3a74ce21e93bfd7/
Highlighted by Newswise: https://www.newswise.com/articles/light-controls-chiral-molecule-structure
Highlighted in mapping ignorance: https://mappingignorance.org/2023/08/29/light-controled-deracemization/?utm_source=rss&utm_medium=rss&utm_campaign=light-controled-deracemization
Highlighted by Nature Asia: http://www.natureasia.com/ko-kr/nature/highlights/122927
© Ryan Gilmour 141. Integrating I(I)/I(III) Catalysis in Reaction Cascade Design Enables the Synthesis of gem-Difluorinated Tetralins from Cyclobutanols
J. Häfliger, L. Ruyet, N. Stübke, C. G. Daniliuc and R. Gilmour, Nature Communications 2023, 14, 3207.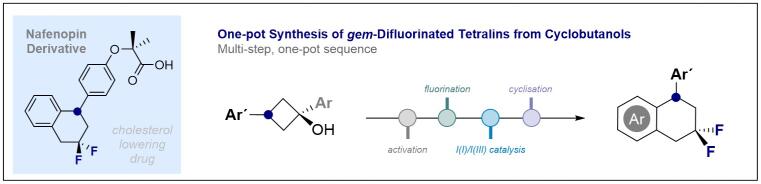
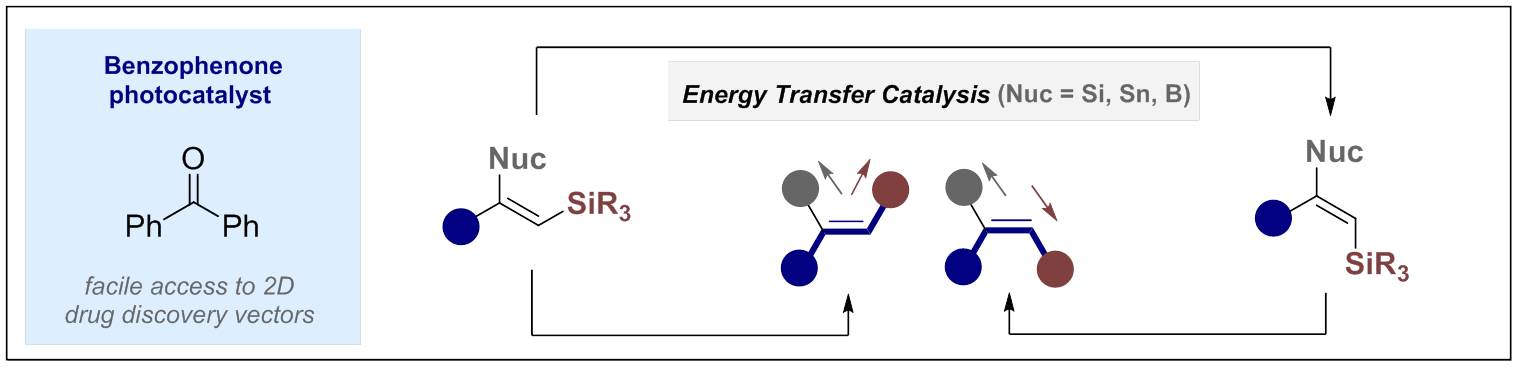
© Ryan Gilmour 140. Direct Observation of Triplet States in the Isomerization of Alkenylboronates by Energy Transfer Catalysis
T. J. B. Zähringer, M. Wienhold, R. Gilmour and C. Kerzig, J. Am. Chem. Soc. 2023, 145, 21576-21586.Highlighted in Synfacts: B. List, K. Kryvous, Synfacts 2023, 19, 0819.

© Ryan Gilmour 139. Forging Medium Rings via I(I)/I(III)-Catalyzed Diene Carbofunctionalization
Y.-J. Yu, J. Häfliger, Z.-X. Wang, C. G. Daniliuc and R. Gilmour, Angew. Chem. Int. Ed. 2023, e202309789.
Open access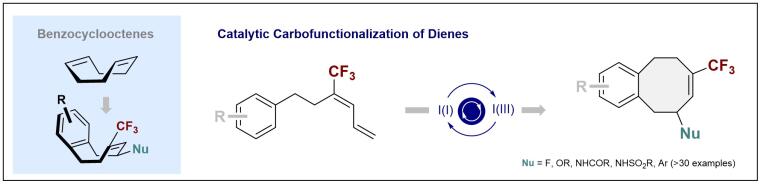
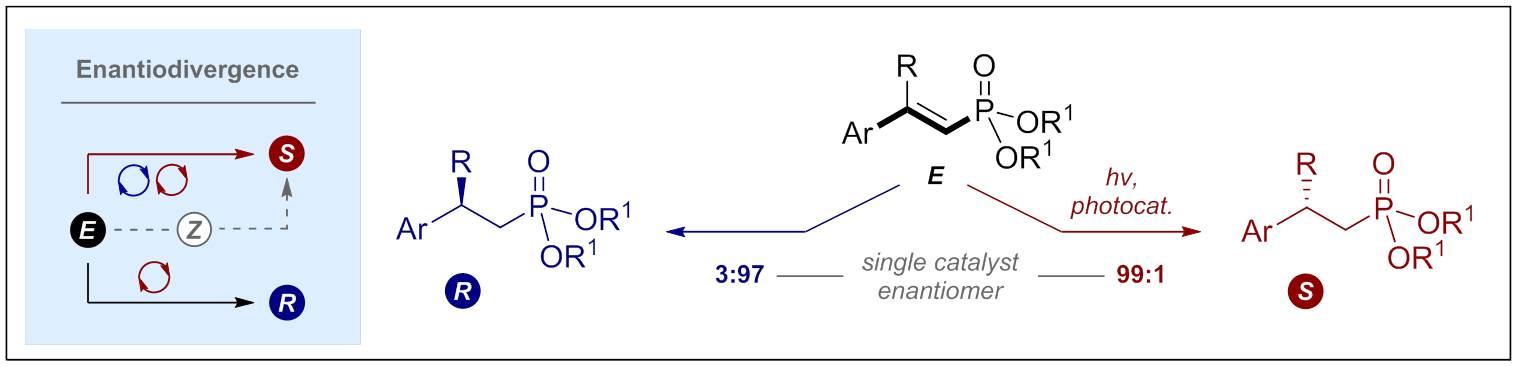
© Ryan Gilmour 138. Geometric Isomerisation of Bifunctional Vinyl Fluoride Linchpins: Stereodivergence in Amide and Polyene Bioisostere Synthesis
M. Wienhold, B. Kweon, C. McLaughlin, M Schmitz, T. J. B. Zähringer, C. G. Daniliuc, C. Kerzig and R. Gilmour, Angew. Chem. Int. Ed. 2023, e202304150.
Hot Paper
Open acces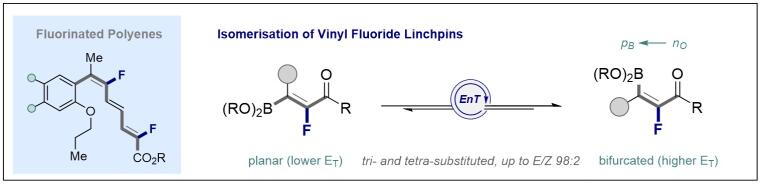
© Ryan Gilmour 137. Fluorinated Carbohydrates for 18F-Positron Emission Tomography (PET)
E. Campbell, C. Jordan and R. Gilmour, Chem. Soc. Rev. 2023, 52, 3599-3626.
Artwork featured on the front cover
© Ryan Gilmour 136. Catalytic, Regioselective 1,4-Fluorodifunctionalization of Dienes
Y.-J. Yu, M. Schäfer, C. G. Daniliuc and R. Gilmour, Angew. Chem. Int. Ed. 2023, 62, e202214906.
Highlighted in Synfacts: B. List, M. M. Poje, Synfacts 2023, 19, 0185.
Open access
© Ryan Gilmour 135. Fluorine-Directed Automated Mannoside Assembly
C. Teschers and R. Gilmour, Angew. Chem. Int. Ed. 2023, 135 e202213304. DOI numbers 10.1002/anie.202213304 and 10.1002/ange.202213304.
Press release: https://www.uni-muenster.de/news/view.php?cmdid=13047&lang=en
Open access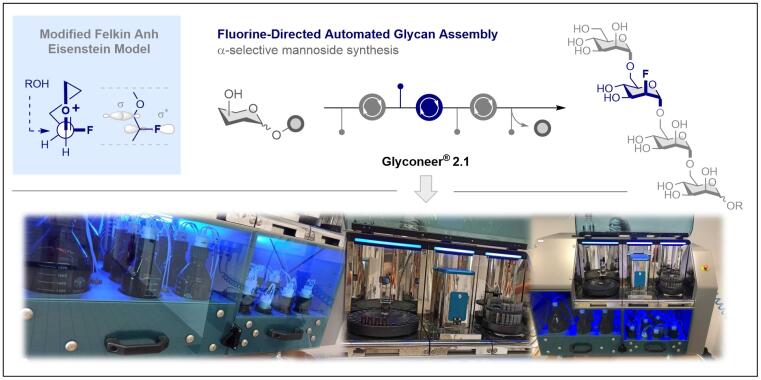
© Ryan Gilmour 134. Fluorocyclisation of Oximes to Isoxazolines via I(I)/I(III) Catalysis
J. Neufeld, C. G. Daniliuc and R. Gilmour, Helv. Chim. Acta 2023, e202200183.
Invited contribution for the special issue dedicated to the memory of the late Prof. Dr. Jack D. Dunitz FRS
Open access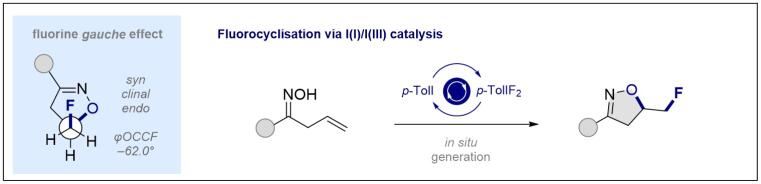
© Ryan Gilmour
2022
133. Skeletal Ring Contractions via I(I)/I(III) Catalysis: Stereoselective Synthesis of cis-α,α-Difluorocyclopropanes
K. Livingstone, K. Siebold, S. Meyer, V. Martín-Heras, C. G. Daniliuc, and R. Gilmour, ACS Catal. 2022, 12, 14507–14516.
Open access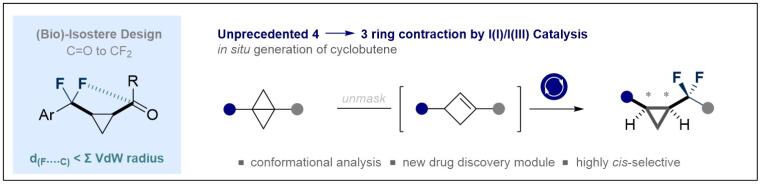
© Ryan Gilmour 132. Organophotocatalytic N–O Bond Cleavage of Weinreb Amides: Mechanism-Guided Evolution of a PET to ConPET Platform
J. Soika, C. McLaughlin, T. Neveselý, C. G. Daniliuc, J. J. Molloy and R. Gilmour, ACS Catal. 2022, 12, 10047-10056.Previously ChemRxiv 2022, DOI: 10.26434/chemrxiv-2022-0fz0p
Highlighted in Org. Proc. Res. Dev.: https://pubs.acs.org/doi/pdf/10.1021/acs.oprd.2c00309
© Ryan Gilmour 131. Cyclopropene Activation via I(I)/I(III) Catalysis: Proof of Principle and Application in Direct Tetrafluorination
S. Meyer, L. Göbel, K. Livingstone, C. Roblick, C. G. Daniliuc and R. Gilmour, Tetrahedron 2022, 132925.
open access
Invited contribution for the special edition dedicated to Prof. Dr. F. Schoenebeck.
© Ryan Gilmour 130. Stereocontrolled Synthesis of Fluorinated Isochromans via Iodine(I)/Iodine(III) Catalysis
J. Häfliger, O. O. Sokolova, M. Lenz, C. G. Daniliuc and R. Gilmour, Angew. Chem. Int. Ed. 2022, e202205277. DOI:10.1002/anie.202205277 and 10.1002/ange.202205277.
Selected as HOT PAPER
© Ryan Gilmour 129. Regio- and Enantioselective Intermolecular Aminofluorination of Alkenes via Iodine(I)/Iodine(III) Catalysis
M. Schäfer, T. Stünkel, C. G. Daniliuc and R. Gilmour, Angew. Chem. Int. Ed. 2022, e202205508. DOI numbers 10.1002/anie.202205508 and 10.1002/ange.202205508
Selected as HOT PAPER
Previously ChemRxiv 2022 DOI: 10.26434/chemrxiv-2022-jnslk
© Ryan Gilmour 128. Advances in the E → Z Isomerization of Alkenes using Small Molecule Photocatalysts
T. Neveselý, M. Wienhold, J. J. Molloy and R. Gilmour, Chem. Rev. 2022, 122, 2650-2694.
Invited contribution for the thematic issue Photochemical Catalytic Processes.
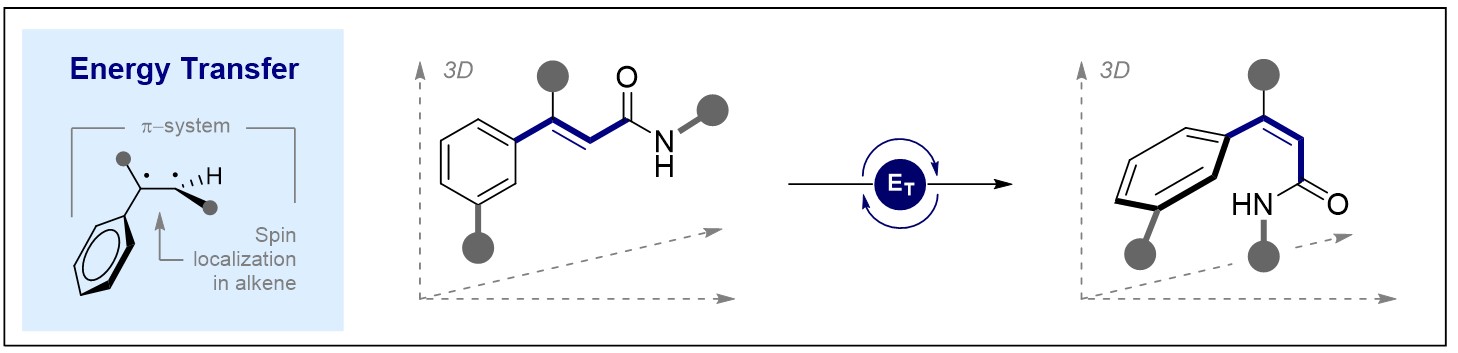
© Ryan Gilmour 127. Leveraging the n→π* Interaction in Alkene Isomerisation by Selective Energy Transfer Catalysis
T. Neveselý, J. J. Molloy, C. McLaughlin, L. Brüss, C. G. Daniliuc and R. Gilmour, Angew. Chem. Int. Ed. 2022, 61, e202113600.
© Ryan Gilmour 126. Leveraging fluorine (stereoelectronic) effects in catalysis in The Chemistry of Organofluorine Compounds
J. Neufeld, K. Livingstone and R. Gilmour, in The Chemistry of Organofluorine Compounds, Patai´s Chemistry of Functional Groups (2022) (Eds. I. Marek, V. Gouverneur, M. Gandelman), Wiley-VCH Verlag GmbH & Co, Weinheim.
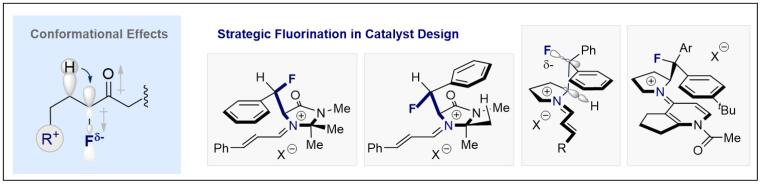
© Ryan Gilmour
2021
125. Enantiodivergent Prenylation via Deconjugative Isomerization
T. Morack, C. Onneken, H. Nakakohara, C. Mück-Lichtenfeld and R. Gilmour, ACS Catal. 2021, 11, 11929–11937.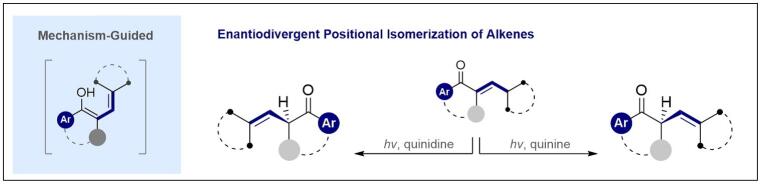
© Ryan Gilmour 124. Illuminating Anti-Hydrozirconation: Controlled Geometric Isomerization of an Organometallic Species
T. Hostmann, T. Nevesely and R. Gilmour, Chem. Sci. 2021, 12, 10643 - 10648.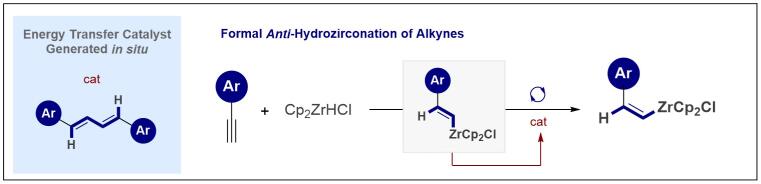
© Ryan Gilmour 123. Expanding Organofluorine Chemical Space: The Design of Chiral Fluorinated Isosteres Enabled by I(I)/I(III) Catalysis
S. Meyer, J. Häfliger and R. Gilmour, Chem. Sci. 2021, 12, 10686 - 10695
Part of the themed collection 2021 Chemical Science HOT article Collection.
© Ryan Gilmour 122. An I(I)/I(III) Catalysis Route to the Heptafluoroisopropyl Group: A Privileged Module in Contemporary Agrochemistry
V. Martín-Heras, C. G. Daniliuc and R. Gilmour, Synthesis 2021, 53, 4203-4212.
Invited contribution for the special edition in honour of Prof. Sarah ReismanSYNTHESIS Best paper Award 2021

© Ryan Gilmour 121. Emerging Fluorinated Motifs: Synthesis, Properties, and Applications (book review)
J. Neufeld and R. Gilmour, Angew. Chem. Int. Ed. 2021, 60, 20592-20593.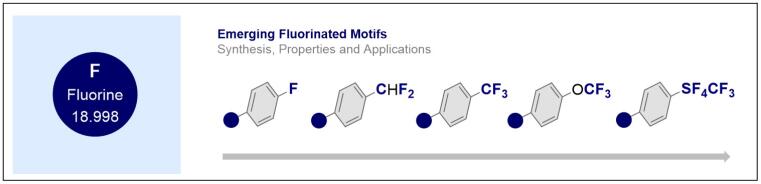
© Ryan Gilmour 120. Difluorination of α-(bromomethyl)styrenes via I(I)/I(III) catalysis: facile access to electrophilic linchpins for drug discovery
J. Häfliger, K. Livingstone, C. G. Daniliuc and R. Gilmour, Chem. Sci. 2021, 12, 6148–6152.
© Ryan Gilmour 119. Trifluorinated Tetralins via I(I)/I(III)-Catalysed Ring Expansion: Programming Conformation by [CH2CH2] → [CF2CHF] Isosterism
J. Neufeld, T. Stünkel, C. Mück-Lichtenfeld, C. G. Daniliuc and R. Gilmour, Angew. Chem. Int. Ed. 2021, 60, 13647-13651.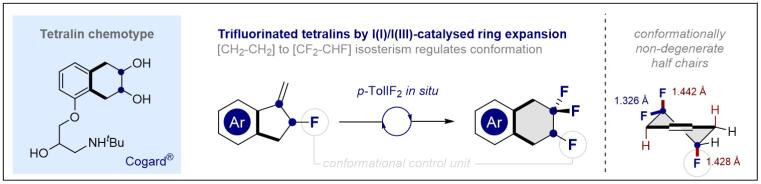
© Ryan Gilmour 118. Oligodendroglial Glycolipids in (Re) Myelination: Implications for Multiple Sclerosis Research
L. Nowack, C. S. Teschers, S. Albrecht, R. Gilmour, Nat. Prod. Rep. 2021, 38, 890-904.
DOI: 10.1039/D0NP00093K.
Artwork featured on the front cover.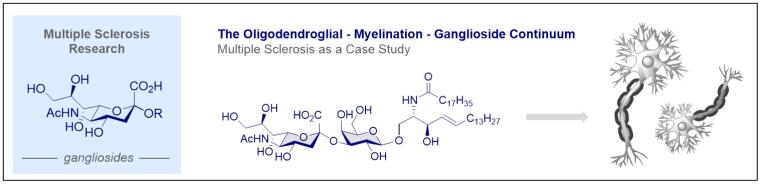
© Ryan Gilmour 117. A Chiral, Pentafluorinated Isopropyl Group via Iodine(I)/(III) Catalysis
S. Meyer, J. Häfliger, M. Schäfer, J. J. Molloy, C. G. Daniliuc, and R. Gilmour, Angew. Chem. Int. Ed. 2021, 60, 6430-6434.
Highlighted in Synfacts: B. List, D. Díaz-Oviedo, Synfacts 2021, 17, 0443.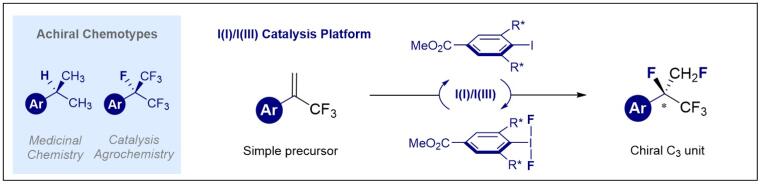
© Ryan Gilmour 116. Coumarins by Direct Annulation: β-Borylacrylates as Ambiphilic C3-Synthons
M. Wienhold, J. J. Molloy, C. G. Daniliuc and R. Gilmour, Angew. Chem. Int. Ed. 2021, 60, 685-689.
Highlighted in Synfacts: P. Knochel, D. Djukanovic, Synfacts 2020, 16, 1444.
© Ryan Gilmour 115. Enhancing Glycan Stability via Site-Selective Fluorination: Modulating Substrate Orientation by Molecular Design
A. Axer, R. P. Jumde, S. Adam, A. Faust, M. Schäfers, M. Fobker, J. Koehnke, A. K. H. Hirsch and R. Gilmour, Chem. Sci. 2021, 12, 1286-1294.
First posted on Chemrxiv 2020, DOI:10.26434/chemrxiv.12656210.
Highlighted as a "hot article" by Chemical Science.
© Ryan Gilmour
2020
113. Cooperative Activation Modes for Catalysis-Based Total Synthesis
S. Meyer and R. Gilmour, Trends Chem. 2020, 2, 959-961.

111. Conformational Analysis of Acyclic α-Fluoro Sulfur Motifs
N. Erdeljac, C. Mück-Lichtenfeld, C. G. Daniliuc and R. Gilmour, Chem. Eur. J. 2020, 26, 13704-13715.

107. Enantioselective Synthesis of 3-Fluorochromanes via I(I)/I(III) Catalysis
J. C. Sarie, C. Thiehoff, J. Neufeld, C. G. Daniliuc and R. Gilmour, Angew. Chem. Int. Ed. 2020, 59, 15069-15075.
Highlighted by ChemViews: https://www.chemistryviews.org/details/ezine/11253871/Enantioselective_3-Fluorochromane_Synthesis.html
Selected as a VIP paper

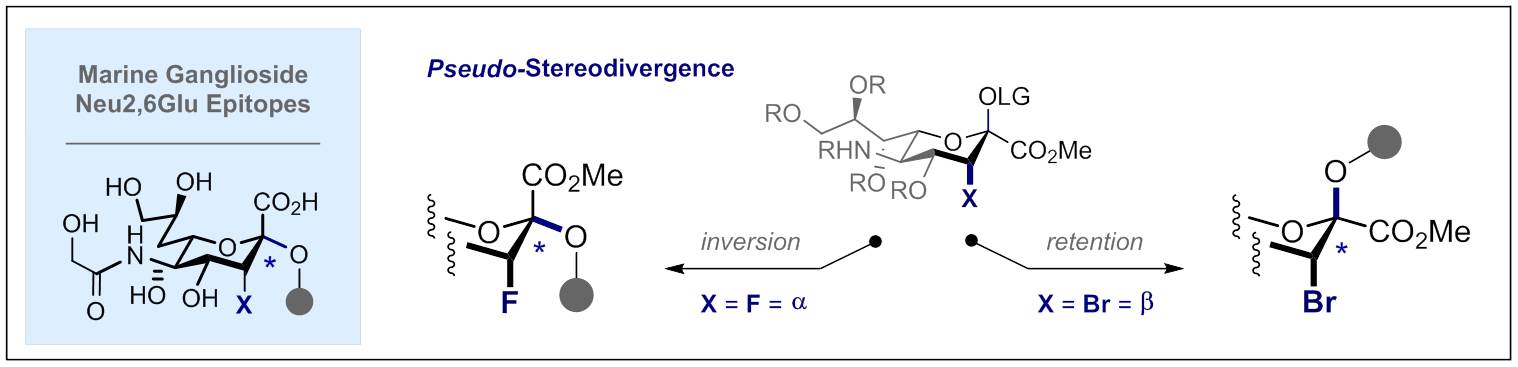
105. Halogen-Directed Chemical Sialylation: Pseudo-Stereodivergent Access to Marine Ganglioside Epitopes
T. Hayashi, A. Axer, G. Kehr, K. Bergander and R. Gilmour, Chem. Sci. 2020, 11, 6527-6531.
2019
102. Sequential Energy Transfer Catalysis: A Cascade Synthesis of Angularly-Fused Dihydrocoumarins
T. Neveselý, C. G. Daniliuc and R. Gilmour, Org. Lett. 2019, 21, 9724-9728.
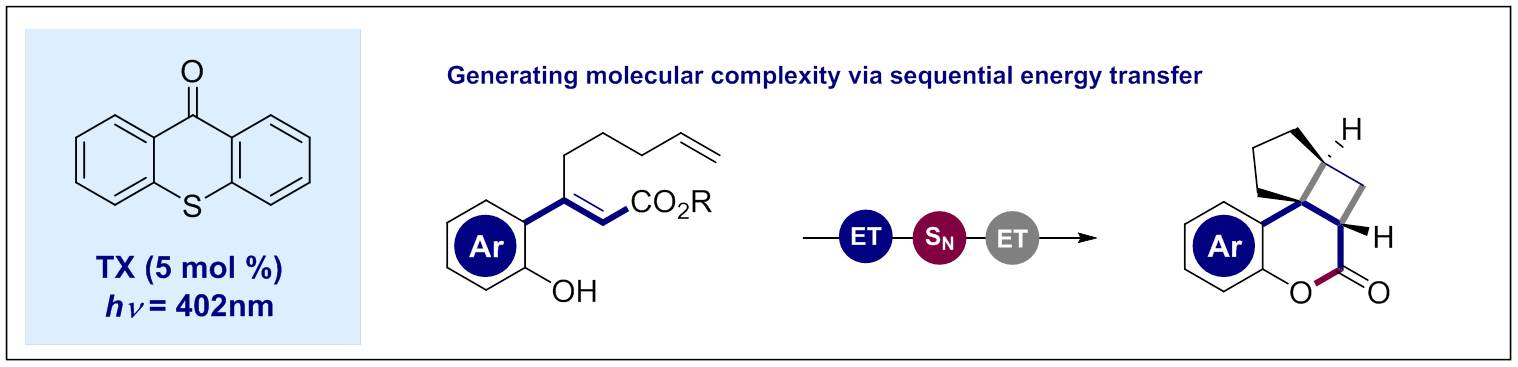
101. Photocatalytic E→Z Isomerization of β-Ionyl Derivatives
K. Livingstone, M. Tenberge, F. Pape, C. G. Daniliuc, C. Jamieson and R. Gilmour, Org. Lett. 2019, 21, 9677-9680.
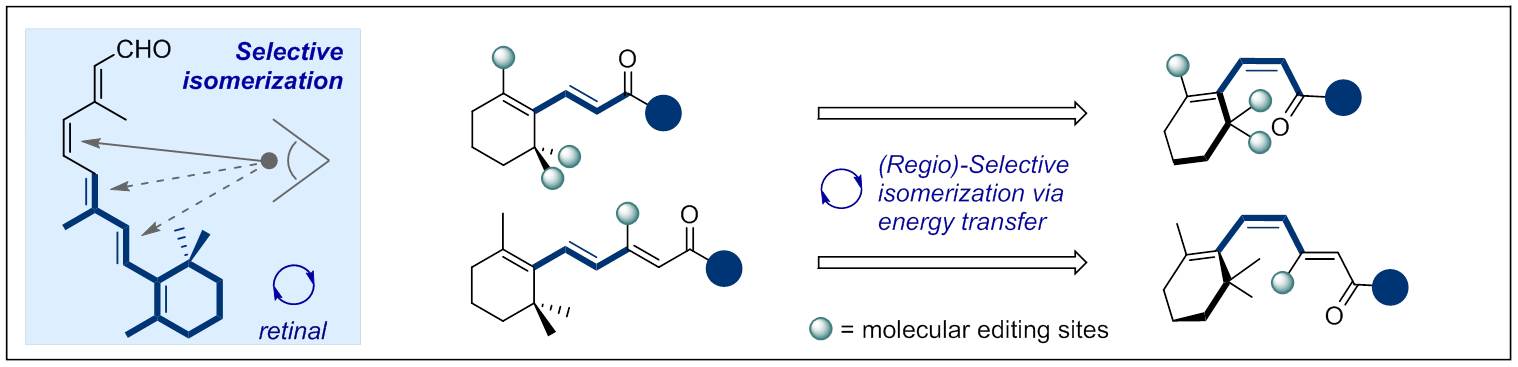
96. Catalytic Vicinal Dichlorination of Unactivated Alkenes
J. C. Sarie, J. Neufeld, C. G. Daniliuc and R. Gilmour, ACS Catal. 2019, 9, 7232-7237.
Highlighted in C&E News "Dichlorination catalyst eschews chlorine gas"
94. Positional and Geometrical Isomerisation of Alkenes: The Pinnacle of Atom EconomyJ. J. Molloy, T. Morack and R. Gilmour, Angew. Chem. 2019, 131, 13789-13800; Angew. Chem. Int. Ed. 2019, 58, 13654-13664.92. Stereospecific α-Sialylation by Site-Selective Fluorination
T. Hayashi, G. Kehr, K. Bergander and R. Gilmour, Angew. Chem. 2019, 131, 3854-3858; Angew. Chem. Int. Ed. 2019, 58, 3814-3818.
91. Bioinspired Radical Stetter Reaction: Radical Umpolung Enabled by Ion-Pair Photocatalysis
T. Morack, C. Mück-Lichtenfeld and R. Gilmour, Angew. Chem. 2019, 131, 1221-1225; Angew. Chem. Int. Ed. 2019, 58, 1208-1212.
Artwork featured on the front cover
Highlighted in Angewandte Chemie: X. -Yu Chen, D. Enders, Angew. Chem. Int. Ed. 2019, DOI: doi.org/10.1002/anie.201902132.
2018
89. A Catalytic Geminal Difluorination of Styrenes for the Construction of Fluorine-Rich Bioisosteres
F. Scheidt, J. Neufeld, M. Schäfer, C. Thiehoff and R. Gilmour, Org. Lett. 2018, 20, 8073–8076.
86. Re-Engineering Chemical Glycosylation: Direct, Metal-Free Anomeric O-Arylation of Unactivated Carbohydrates
N. Lucchetti and R. Gilmour, Chem. Eur. J. 2018, 24, 16266-16270.
85. Informing Molecular Design by Stereoelectronic Theory: The Fluorine Gauche Effect in Catalysis
M. Aufiero and R. Gilmour, Acc. Chem. Res. 2018, 51, 1701-1710.
81. Conformational Control Enabled by the Fluorine Gauche Effect in a Model of the β2-AR Agonist Salbutamol (VentolinTM)
C. S. Teschers, C. G. Daniliuc, G. Kehr and R. Gilmour, J. Fluorine Chem. 2018, doi.org/10.1016/j.jfluchem.2018.02.007.
Invited Contribution for the special edition in memory of Prof. George Olah
80. Vitamin Catalysis: Direct, Photocatalytic Synthesis of Benzocoumarins via (–)-Riboflavin-Mediated Electron Transfer
T. Morack, J. B. Metternich and R. Gilmour, Org. Lett. 2018, 20, 1316-1319.
76. Spatiotemporal Control of Pre-Existing Alkene Geometry: A Bio-Inspired Route to 4-Trifluoromethyl-2H-Chromenes
S. Faßbender, J. B. Metternich and R. Gilmour, Org. Lett. 2018, 20, 724-727.
74. Harnessing the Maltodextrin Transport Mechanism for Targeted Bacterial Imaging: Structural Requirements for Improved in vivo Stability in Tracer Design
A. Axer, S. Hermann, G. Kehr, D. Clases, U. Karst, L. Fischer-Riepe, J. Roth, M. Fobker, M. Schäfers, A. Faust* and R. Gilmour*, ChemMedChem 2018, 13, 241-250.
Included in the ChemMedChem Virtual Issue on Antibacterials, available at bit.ly/cmdcantibac18
Artwork featured on the front cover
2017
73. Lewis Base Catalysis in Organic Synthesis. 3 Volume Set. Edited by Edwin Vedejs and Scott E. Denmark (book review)
M. Aufiero, R. Gilmour, Angew. Chem. Int. Ed. 2017, 56, 12045.
© Ryan Gilmour 72. Deconstructing the Catalytic, Vicinal Difluorination of Alkenes: HF-Free Synthesis and Structural Study of p-TolIF2
Jérôme C. Sarie, Christian Thiehoff, Richard J. Mudd, Constantin G. Daniliuc, Gerald Kehr and Ryan Gilmour, J. Org. Chem. 2017, 82, 11792–11798.

© Ryan Gilmour 71. Photocatalytic E → Z Isomerization of Polarized Alkenes Inspired by the Visual Cycle: Mechanistic Dichotomy and Origin of Selectivity
Jan B. Metternich, Denis G. Artiukhin, Mareike C. Holland, Maximilian von Bremen-Kühne, Johannes Neugebauer and Ryan Gilmour, J. Org. Chem. 2017, 82, 9955–9977.
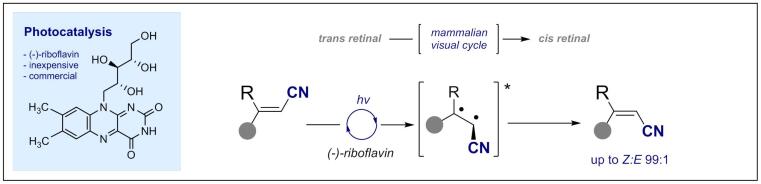
© Ryan Gilmour 70. The Fluorine Gauche Effect: A Brief History
C. Thiehoff, Y. P. Rey, R. Gilmour, Isr. J. Chem. 2017, 57, 92-100.
Invited for the "Rosarium Philosophorum on Structural Chemistry", in honour of Prof. Dr. Jack D. Dunitz FRS. Open Access
© Ryan Gilmour 69. Quantitative Profiling of the Heavy Atom Effect in BODIPY Dyes: Correlating Initial Rates, Atomic Numbers and 1O2 Quantum Yields
Y. P. Rey, D. G. Abradelo, N. Santschi, C. A. Strassert and R. Gilmour, Eur. J. Org. Chem. 2017, accepted for publication.
Invited contribution for the special edition on photocatalysis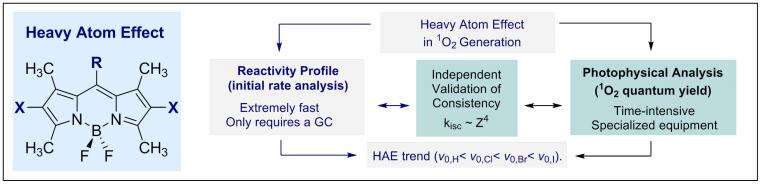
© Ryan Gilmour 68. Emulating Natural Product Conformation by Cooperative, Non-Covalent Fluorine Interactions
F. Scheidt, P. Selter, N. Santschi, M. C. Holland, D. V. Dudenko, C. Daniliuc, C. Mück-Lichtenfeld, M. R. Hansen and R. Gilmour, Chem. Eur. J. 2017, accepted for publication.
Invited contribution for the special edition SFB 858 Münster "Synergistic Effects in Chemistry"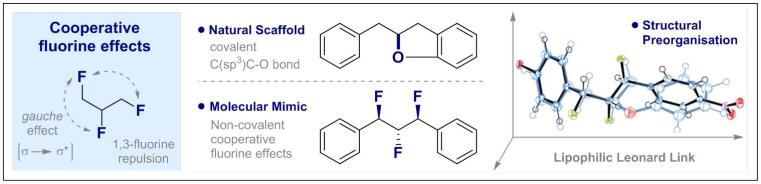
© Ryan Gilmour
2016
67. Photocatalytic E → Z Isomerization of Alkenes
J. B. Metternich and R. Gilmour, Synlett 2016, 27, 2541-2552.
Invited perspective
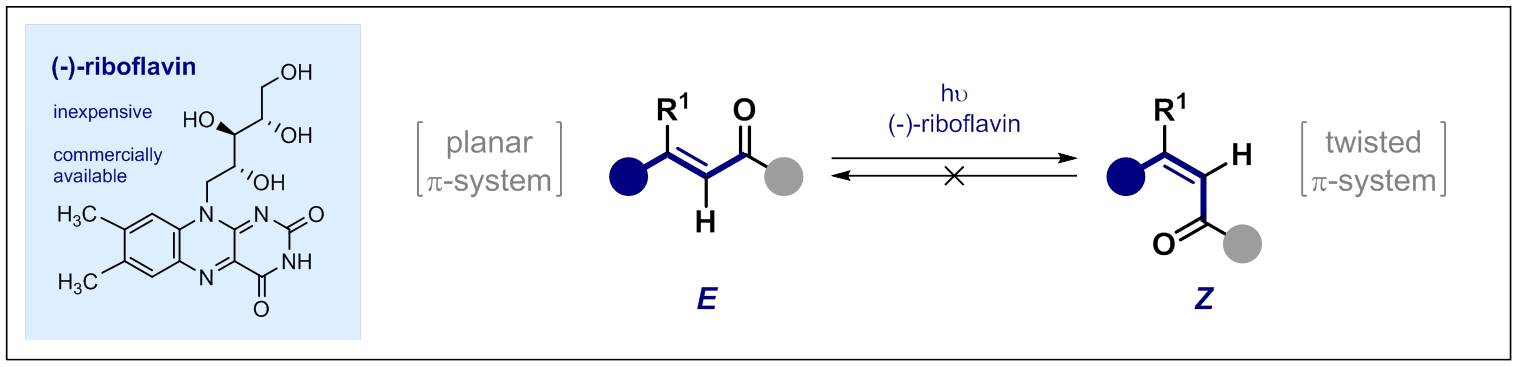
66. Catalytic, Vicinal Difluorination of Olefins: Creating a Hybrid, Chiral Bioisostere of the Trifluoromethyl and Ethyl Groups
I. G. Molnár, C. Thiehoff, M. C. Holland and R. Gilmour, ACS Catal. 2016, 6, 7167–7173.
Invited contribution
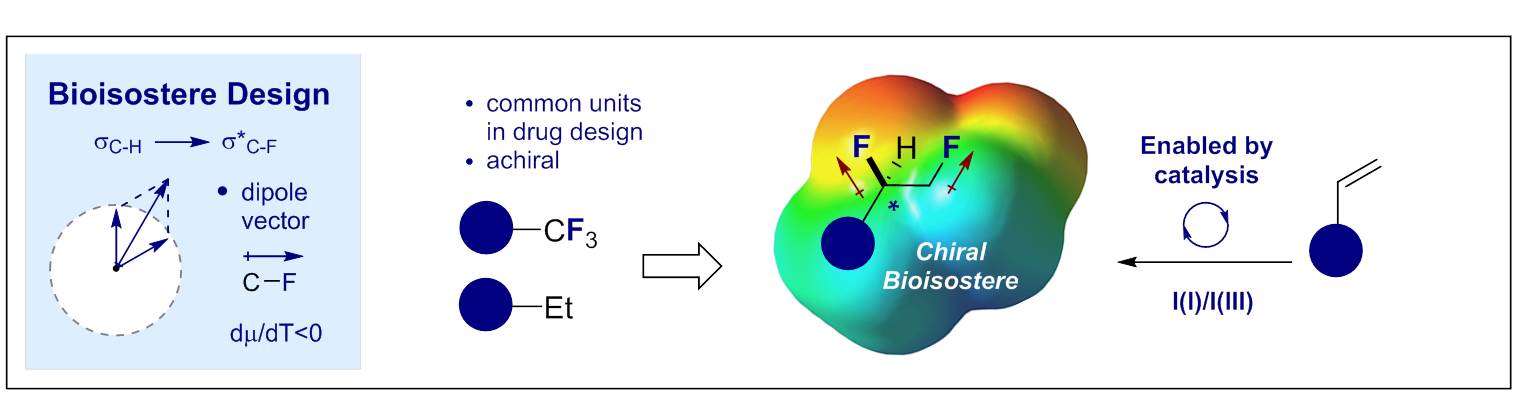
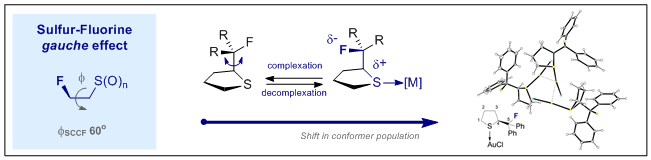
65. The Sulfur-Fluorine Gauche Effect in Coinage Metal Complexes: Augmenting Conformational Equilibria by Complexation
N. Santschi, C. Thiehoff, M. C. Holland, C. G. Daniliuc, K. N. Houk and R. Gilmour,
Organometallics 2016, 35, 3040–3044
64. Catalytic Difluorination of Olefins
I. G. Molnár and R. Gilmour, J. Am. Chem. Soc. 2016, 138, 5004–5007.
This article is featured in JACS spotlight!
This work is highlighted in ACS C&E News
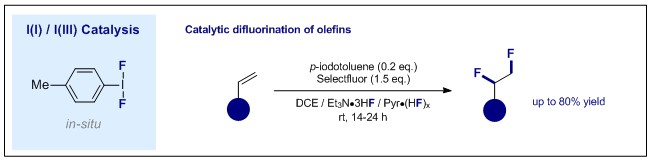
63. A “One Photocatalyst, n Activation Modes” Strategy for Cascade Catalysis: Emulating Coumarin Biosynthesis with (–)-Riboflavin
J. B. Metternich and R. Gilmour, J. Am. Chem. Soc. 2016, 138, 1040–1045.
"This article is featured in JACS spotlight!"
"Artwork featured on the front cover"
This work is highlighted in ACS C&E News
"Highlighted in Synfacts issue 04/2016"
62. Importance of Intermolecular Hydrogen Bonding for the Stereochemical Control of Allene-Enone (3+2) Annulations Catalyzed by a Bifunctional, Amino Acid Derived Phosphine Catalyst
M. C. Holland, R. Gilmour and K. N. Houk, Angew. Chem. Int. Ed. 2016, 128, 2062-2067.
61. Fluorine-Directed 1,2-trans Glycosylation of Rare Sugars
N. Aiguabella, M. C. Holland and R. Gilmour, Org. Biomol. Chem. 2016, 14, 5534-5538.
Invited contribution for the special New Talent Themed Edition.
60. The Influence of Electronic Perturbations on the Sulfur-Fluorine Gauche Effect
C. Thiehoff, L. Schifferer, C. G. Daniliuc, N. Santschi and R. Gilmour, J. Fluorine Chem. 2016, 182, 121-126.
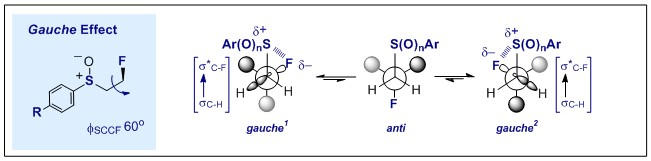
59. Organocatalysis Intermediates as Platforms to Study Non-Covalent Interactions: Integrating Fluorine Gauche Effects in Iminium Systems to Facilitate Acyclic Conformational Control
I. G. Molnár, M. C. Holland, C. Daniliuc, K. N. Houk and R. Gilmour, Synlett 2016, 27, 1051–1055.
Invited contribution for the Special Cluster Edition on Non-covalent interacts in Catalysis.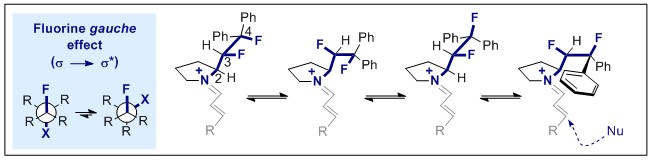
2015
58. A Bio-Inspired, Catalytic E→Z Isomerization of Activated Olefins J. B. Metternich and R. Gilmour, J. Am. Chem. Soc. 2015, 137, 11254-11257.
This work is highlighted in ACS C&E News (http://cen.acs.org/articles/93/i36/Easier-Way-E-Z.html?type=paidArticleContent)
Royal Society of Chemistry "Chemistry World" (http://www.rsc.org/chemistryworld/2015/09/riboflavin-catalysed-alkene-isomerisation)
Research in Germany (http://www.research-in-germany.org/de.html)
Informationsdienst Wissenschaft in German and English (https://idw-online.de/de/)
WWU Press release (http://www.uni-muenster.de/news/view.php?cmdid=7859
Highlighted by the ACS as "molecule of the week" (http://www.acs.org/content/acs/en/molecule-of-the-week/archive/r/riboflavin.html?cid=home_motw)

55. A Comparative Analysis of Fluorine-Directed Glycosylation Selectivity:
Interrogating C2 [OH → F] Substitution in D-Glucose and D-GalactoseN. Santschi and R. Gilmour, Eur. J. Org. Chem. 2015, 32, 6983-6987.
"Artwork featured on the front cover"

53. Delineating the Physical Organic Profile of the 6-Fluoro Glycosyl Donor
N. Santschi, N. Aiguabella, V. Lewe and R. Gilmour, J. Fluorine Chem. 2015, 96-101.
Invited contribution for the special edition in honour of Prof. V. Gouverneur.

52. Angewandte Chemie Author Profile
R. Gilmour, Angew. Chem. Int. Ed. 2015, 54, 11012.

51. Can Acyclic Conformational Control be Achieved via a Sulfur-Fluorine Gauche Effect?
C. Thiehoff, M. C. Holland, C. Daniliuc, K. N. Houk and R. Gilmour, Chem. Sci. 2015, 6, 3565-3571.

50. Aromatic Interactions in Organocatalyst Design: Augmenting Selectivity Reversal in Iminium Ion Activation
M. C. Holland, J. B. Metternich, C. Daniliuc, W. B. Schweizer and R. Gilmour, Chem. Eur. J. 2015, 21. 10031-10038."Artwork featured on the front cover"
Selected as a Hot Paper. Featured on the journal Facebook page.

49. A Janus Cyclohexane Ring
N. Santschi and R. Gilmour, Nature Chemistry, 2015, 7, 467-468. (News and Views).

48. Stereochemical Bias Introduced During RNA Synthesis Modulates the Activity of Phosphorothioate siRNAs H. Jahns, M. Roos, J. Imig, F. Baumann, Y. Wang, R. Gilmour and J. Hall, Nature Communications, 2015, 6:6317 doi:10.1038/ncomms7317.

47. Deconstructing Covalent Organocatalysis Reactions
M. C. Holland and R. Gilmour, Angew. Chem. 2015, 127, 3934-3943; Angew. Chem. Int. Ed. 2015, 54, 3862-3871.

46. Cation-π Interactions in Iminium Ion Activation: Correlating Quadrupole Moment & Enantioselectivity
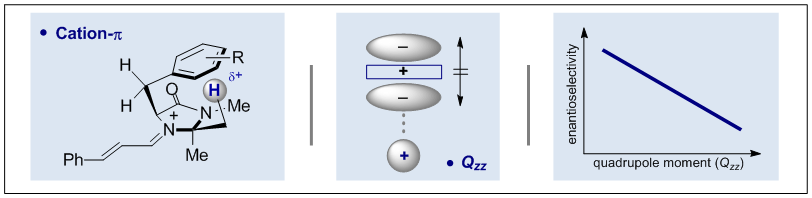
M. C. Holland, J. B. Metternich, C. Mück-Lichtenfeld and R. Gilmour, Chem. Commun. 2015, 51, 5322-5325."Invited for the Emerging Investigators Issue 2015"
"Artwork featured on the front cover"
45. Adsorption and Stability of Chiral Modifiers based on 1-(1-naphthyl)-ethylamine for Pt Catalysed Heterogeneous Asymmetric Hydrogenations
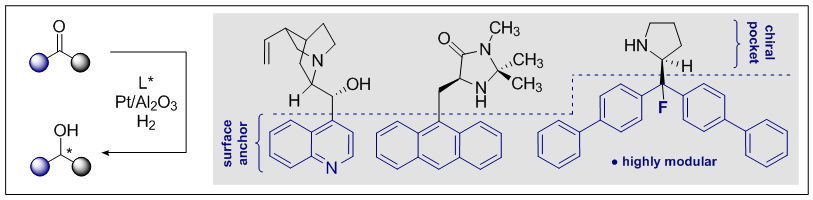
F. Meemken, T. Steiger, M. Holland, R. Gilmour, K. Hungerbühler and A. Baiker, Catal. Sci. Technol. 2015, 5, 705-715.

44. Chiral Imidazolidinone and Proline-Derived Surface Modifiers for the Pt-Catalyzed Asymmetric Hydrogenation of Activated Ketones
M. Holland, F. Meemken, A. Baiker and R. Gilmour, J. Mol. Catal. A: Chem. 2015, 396, 335-345.
2014
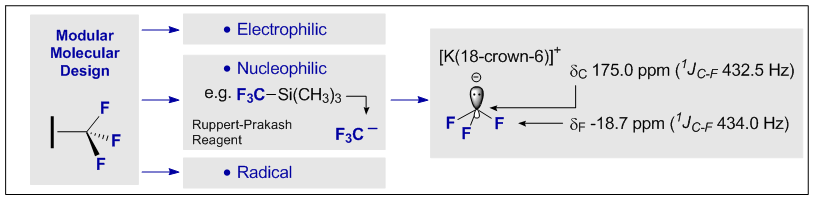
43. The (not so) Ephemeral Trifluoromethanide Anion
N. Santschi and R. Gilmour, Angew. Chem. Int. Ed. 2014, 53, 11414-11415.
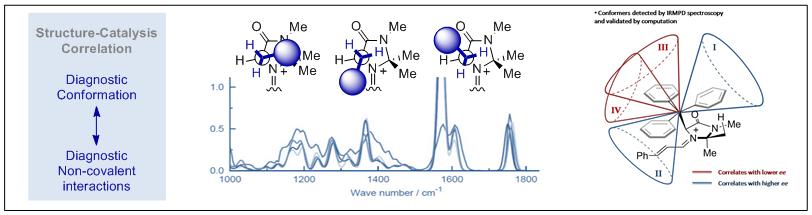
42. Infrared Multiphoton Dissociation (IRMPD) Spectroscopic Analysis of Non-Covalent Interactions in Organocatalysis
M. C. Holland, G. Berden, J. Oomens, A. J. H. M. Meijer, M. Schäfer and R. Gilmour,
Eur. J. Org. Chem. 2014, 26, 5675-5680.
41. Chimia "Fluorine Chemistry": Editorial
D. Seebach and R. Gilmour, Chimia 2014, 345.40. Molecular Design Exploiting a Fluorine Gauche Effect as a Stereoelectronic Trigger
Y. P. Rey, L. E. Zimmer, C. Sparr, E.-M. Tanzer, W. B. Schweizer, H. M. Senn, S. Lakhdar and R. Gilmour, Eur. J. Org. Chem. 2014, 1202-1211.

39. Enantioselective Aziridination of Cyclic Enals Facilitated by the Fluorine-Iminium Ion Gauche Effect
I. G. Molnar, E.-M. Tanzer, C. Daniliuc and R. Gilmour, Chem. Eur. J. 2014, 20, 794-800.
38. The Fluorine Gauche Effect in Molecular Design: A Personal Perspective
Y. P. Rey and R. Gilmour, Seminars in Organic Synthesis (book chapter), 2014, 90-98.

2013
37. Modulating NHC Catalysis with Fluorine
Y. Rey and R. Gilmour, Beilstein J. Org. Chem. 2013, 9, 2812-2820
*Organofluorine Themed Edition

36. Non-Covalent Interactions in Organocatalysis: Modulating Conformational Diversity and Reactivity in the MacMillan Catalyst.
M. C. Holland, S. Paul, W. B. Schweizer, K. Bergander, C. Mück-Lichtenfeld, S. Lakhdar, H. Mayr and R. Gilmour, Angew. Chem. 2013, 125, 8125-8129; Angew. Chem. Int. Ed. 2013, 52, 7967-7971.

35. The Fluorine-NHC Gauche Effect: A Structural and Computational Study.
S. Paul, W. B. Schweizer, G. Rugg, H. M. Senn and R. Gilmour, Tetrahedron 2013, 69, 5647-5659.

34. Happy 90th Birthday: Professor Jack David Dunitz FRS, the Professor's Professor.
W. B. Schweizer and R. Gilmour, Helv. Chim. Acta 2013, 96, 539-544.
2012
33. Fluorinated Organocatalysts for the Enantioselective Epoxidation of Enals: Molecular Pre-organisation by the Fluorine-Iminium Ion Gauche Effect.
E.-M. Tanzer, L. E. Zimmer, W. B. Schweizer and R. Gilmour, Chem. Eur. J. 2012, 18, 11334.
Selected as a VIP Paper by the referees.
32. Exploiting Fluorine Conformational Effects in Organocatalyst Design:The Fluorine-Iminium Ion Gauche Effect.
C. Sparr, L. E. Zimmer and R. Gilmour, (2011) in Asymmetric Syntheses. More Methods and Applications (Eds. S. Bräse and M. Christmann), Wiley-VCH Verlag GmbH & Co, Weinheim, 117-124.
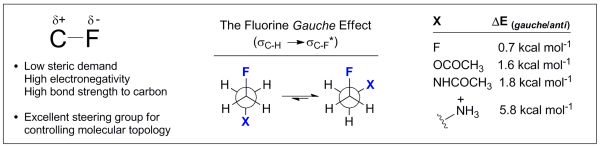
31. Fluorine-Directed β-Galactosylation: Chemical Glycosylation Development by Molecular Editing.
E. Durantie, C. Bucher and R. Gilmour, Chem. Eur. J. 2012, 18, 8208.

30. Fluorinated Cinchona Alkaloids in Phase Transfer Catalysis: Controlling Internal Rotation by a Fluorine-Ammonium Ion Gauche Effect (ØNCCF).
E.-M. Tanzer and R. Gilmour, Chem. Eur. J. 2012, 18, 2006.

29. Fundamental Insights into the Enantioselectivity of Hydrogenations on Cinchona-Modified Platinum and Palladium.
E. Schmidt, C. Bucher, G. Santarossa, T. Mallat, R. Gilmour and A. Baiker, J. Catal. 2012, 289, 238.

28. d,l-Ribose Crystal Structures: the Glass-Crystal Transformation.
G. Zandomeneghi,* W. B. Schweizer,* R. Gilmour,* B. H. Meier,* and J. D. Dunitz,*Helv. Chim. Acta (D. Seebach 75th Birthday Edition) 2012, 95, 1687.

27. The YFM 2012 – Emerging Research and Established Needs: Fund Raising, Publishing and Career Advancement.
B. Winter-Werner, F. Schoenebeck and R. Gilmour, Chimia 2012, 66, 717.
2011
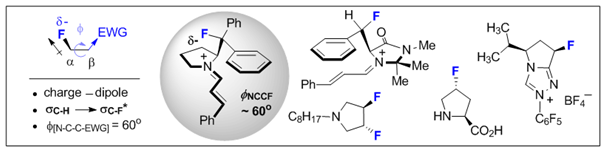
26. Fluorine Conformational Effects in Organocatalysis: An Emerging Strategy for Molecular Design.
L. E. Zimmer, C. Sparr and R. Gilmour, Angew. Chem. 2011, 123, 12062; Angew. Chem. Int. Ed. 2011, 50, 11860.
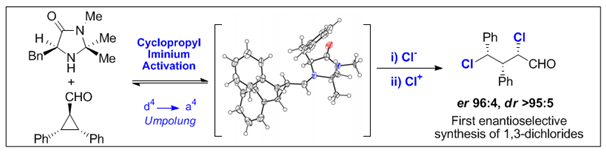
25. Cyclopropyl Iminium Activation: Reactivity Umpolung in Enantioselective Organocatalytic Reaction Design.
C. Sparr and R. Gilmour, Angew. Chem. 2011, 123, 8541-8545; Angew. Chem. Int. Ed. 2011, 50, 8391-8395. Selected as a Hot Paper by Angewandte Chemie.
24. Theoretical and X-ray Crystallographic Evidence of a Fluorine-Imine Gauche Effect: An Addendum to Dunathan’s Stereoelectronic Hypothesis.
C. Sparr, E. Salamanova, W. B. Schweizer, H. M. Senn and R. Gilmour, Chem. Eur. J. 2011, 17, 8850.

23. The 46th EUCHEM Conference on Stereochemistry (Bürgenstock Conference 2011).
L. J. Prins and R. Gilmour, Chimia, 2011, 65, 612.22. Homage to Vladimir Prelog.
R. Gilmour, Chemistry World 2011, (book review).21. Steering Glycosylation with the C-F Bond.
C. Bucher and R. Gilmour, Synlett 2011, 1043.
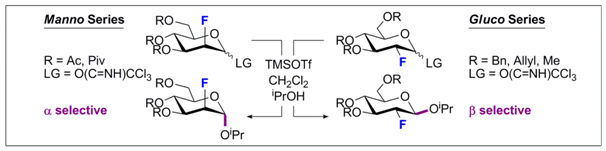
20. A Modular Synthesis of Fluorinated, Chiral Polar Lipids.
C. Bucher and R. Gilmour, Synthesis 2011, 549.

2010
19. A Novel Fluorinated Au(I) N-Heterocyclic Carbene Complex: Exploiting Fluorine Stereoelectronic Effects to Control Molecular Topology.
S. Paul, W. B. Schweizer, M.-O. Ebert, and R. Gilmour, Organometallics 2010, 29, 4424.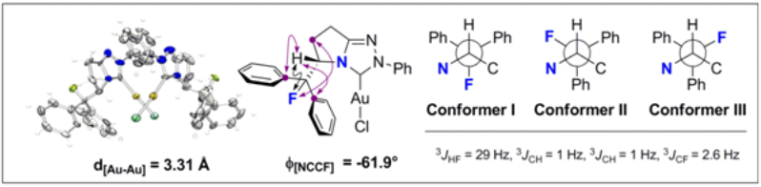
© Ryan Gilmour 18. Fluorine-Directed Glycosylation.
C. Bucher and R. Gilmour, Angew. Chem. 2010, 122, 8906; Angew. Chem. Int. Ed. 2010, 49, 8724.
© Ryan Gilmour 17. Fluoro-Organocatalysts: Conformer Equivalents as a Tool for Mechanistic Studies.
C. Sparr and R. Gilmour, Angew. Chem. 2010, 122, 6670; Angew. Chem. Int. Ed. 2010, 49, 6520.
Selected as a Hot Paper by Angewandte Chemie.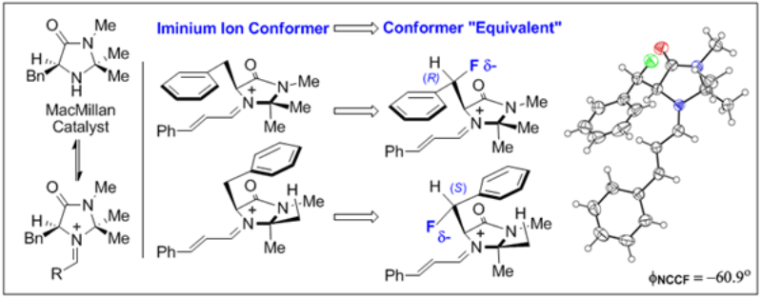
© Ryan Gilmour 16. A Novel Class of Fluorinated Cinchona Alkaloids as Surface Modifiers for the Heterogeneous Platinum-Catalysed Enantioselective Hydrogenation of α-Ketoesters.
C. Bucher, C. Mondelli, A. Baiker and R. Gilmour, J. Mol. Catal. A: Chem. 2010, 327, 87.
© Ryan Gilmour 15. The Crystal Structure of D-Ribose – At Last!
D. Šišak, L. B. McCusker, G. Zandomeneghi, B. Meier, D. Bläser, R. Boese, W. B. Schweizer, R. Gilmour and J. D. Dunitz, Angew. Chem. 2010, 122, 4605; Angew. Chem. Int. Ed. 2010, 49, 4503.
© Ryan Gilmour 14. Stereochemical Models for Discussing Additions to α,β-Unsaturated Aldehydes Organocatalysed by Diarylprolinol or Imidazolidinone Derivatives – is there an E/Z-Dilemma?
D. Seebach,* R. Gilmour,* U. Grošelj, G. Deniau, C. Sparr, M.-O. Ebert and A. K. Beck, Helv. Chim. Acta 2010, 93, 603.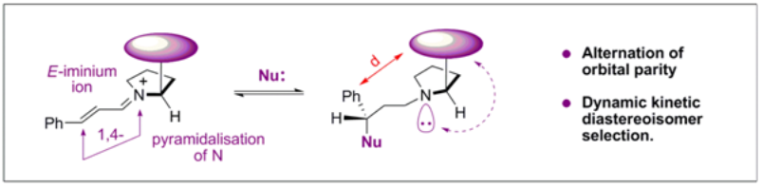
© Ryan Gilmour 13. A Synthesis of (S)-2-(Fluorodiphenylmethyl)-Pyrrolidine: A Novel Organocatalyst for the Stereoselective Epoxidation of α,β-Unsaturated Aldehydes.
C. Sparr, E.-M. Tanzer, J. Bachmann and R. Gilmour, Synthesis 2010, 1394.
© Ryan Gilmour
2009
12. Fluorinated Quinine Alkaloids: Synthesis, X-ray Structure Analysis and Anti- Malarial Parasite Chemotherapy.
C. Bucher, C. Sparr, W. B. Schweizer and R. Gilmour, Chem. Eur. J. 2009, 15, 7637.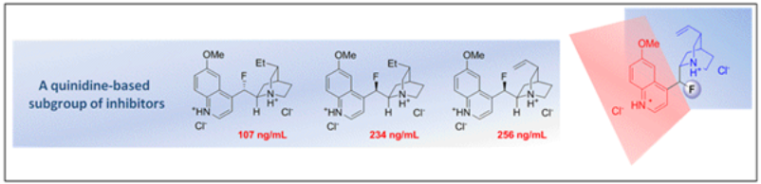
© Ryan Gilmour 11. The Fluorine-Iminium Ion Gauche Effect: Proof of Principle and Application to Asymmetric Organocatalysis.
C. Sparr, W. B. Schweizer, H. M. Senn and R. Gilmour, Angew. Chem. 2009, 121, 3111; Angew. Chem. Int. Ed. 2009, 48, 3065.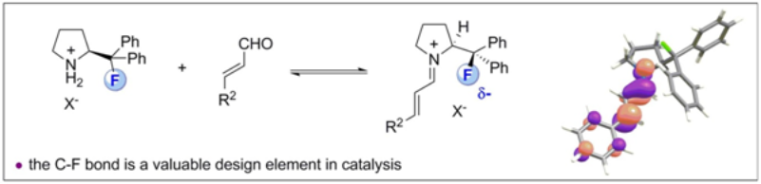
© Ryan Gilmour 10. Total Syntheses of Amphidinolides B1, B4, G1, H1 and Structural Revision of Amphidinolide H2.
A. Fürstner, L. C. Bouchez, L. Morency, J.-A. Funel, V. Liepins, F.-H. Poree, R. Gilmour, D. Laurich, F. Beaufils and M. Tamiya, Chem. Eur. J. 2009, 15, 3983.9. The Handbook of Chemical Glycosylation: Advances in Stereoselectivity and Therapeutic Relevance.
R. Gilmour and P. H. Seeberger, J. Am. Chem. Soc. 2008, 130, 11241.
8. Fast and Efficient Radical Based-Reduction and Hydrosilylation Reactions in a Microreactor Using Tris(Trimethylsilyl)silane.
A. Odedra, K. Geyer, T. Gustafsson, R. Gilmour and P. H. Seeberger, Chem. Commun. 2008, 3025.
7. Fluorination Reactions made Safe by Microreactors.
T. Gustaffson, R. Gilmour and P. H. Seeberger, Chem. Commun. 2008, 3022.
6. The Development of a High-Throughput, Microarray-Based Synthesis of Natural Product Analogues via in Vitro Pathway Construction.
R. Gilmour and P. H. Seeberger, ChemTracts-Organic Chemistry 2007, 191.
5. Structural Diversity in Imidazolidinone Organocatalysts: A Synchrotron and Computational Study.
J. C. Burley, R. Gilmour, T. J. Prior and G. M. Day, Acta Cryst. 2008, C64, o10.
4. The Total Synthesis of Amphidinolide H and G.
A. Fürstner, L. C. Bouchez, J. -A. Funel, V. Liepins, F.–H. Porée, R. Gilmour, F. Beaufils, D. Laurich and M. Tamiya, Angew. Chem. 2007, 119, 9425; Angew. Chem. Int. Ed. 2007, 46, 9265.
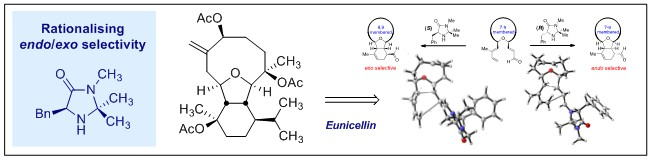
3. An Organocatalytic Approach to the Core of Eunicellin.
R. Gilmour, T. J. Prior, J. W. Burton and A. B. Holmes, Chem. Commun. 2007, 3954.
2. The Synthesis and Biological Evaluation of Novel Eunicellin Analogues.
J. E. P. Davidson, R. Gilmour, S. Ducki, J. E. Davies, R. Green, J. W. Burton and A. B. Holmes, Synlett 2004, 8, 1434.
1. Synthetic Studies Related to Diketopyrrolopyrrole (DPP) Pigments. Part 1: The Search for Alkenyl DPPs. Unsaturated Nitriles in Standard DPP Syntheses: A Novel Cyclopenta[c]pyrrolone Chromophore.
J. H. Morton, R. Gilmour, D. M. Smith, P. Lightfoot, A. M. Z. Slawin, and E. J. MacLean, Tetrahedron 2002, 58, 5547.