PROJECT A15
Structural Insights into the Complex Interplay between Latrotoxin and the Cell Adhesion Receptors Neurexin and Latrophilin
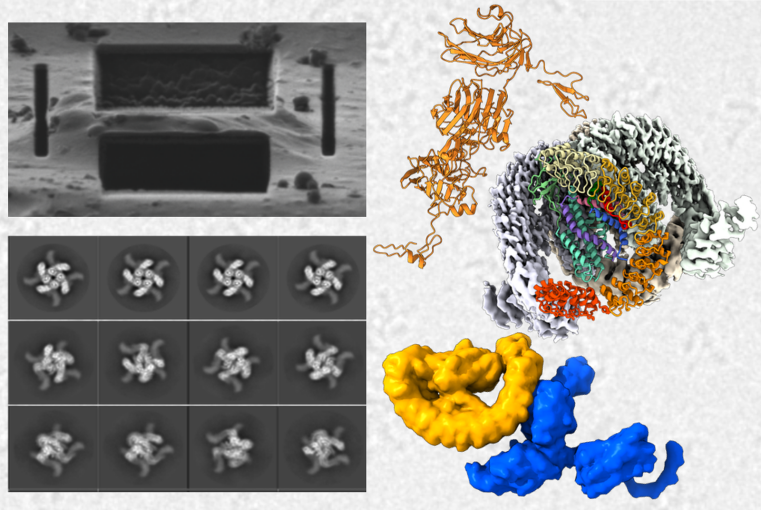
Synaptic adhesion complexes are multifunctional molecules that coordinate different aspects of synaptic function, for example through signaling or interaction with other proteins. They are implicated in a plethora of phychiatric disorders and it comes to no surprise that they are often the main target of nature’s poisons, for example venom-derived neurotoxins. Along this line, the cell adhesion GPCR Latrophilin 1 (LPHN1) and a major class of presynaptic organizers, neurexins (NRXNs), were both first discovered as receptors of the potent neurotoxin α-Latrotoxin (α-LTX). Both were suggested later to interact with each other with high affinity, forming an intriguing intercellular adhesion complex. The interaction of the toxin with these receptors induces insertion of the toxin into the presynaptic membrane, neurotransmitter release and synapse degeneration. α-LTX has been therefore widely used as a molecular tool to study the exocytosis of synaptic vesicles and its actions are considered precisely the opposite of those of botulinum and tetanus toxins, both of which inhibit instead of activating the same secretory apparatus.
These processes at the presynaptic membrane are not understood, as we are lacking high-resolution structures for α-LTX, LPHN1, and NRXNs. Our project aims to unravel the complex interplay between NRXN, LPHN1, and LTX and shed light into the underlying molecular mechanisms. This will be achieved through high-resolution visualization and structural studies of the respective complexes, both in vitro and in situ, predominantly utilizing advanced cryoEM methods.


