Optimal Voltage Window for Lithium-ion Batteries with Lithium/Manganese-rich Layered Oxide Cathodes
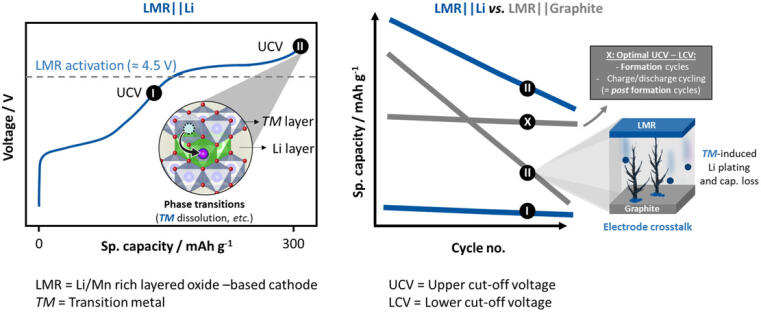
Lithium-/manganese-rich layered oxides (LMR) are considered a promising cathode material for high-energy lithium-ion batteries. Compared to cathodes based on lithium nickel manganese cobalt oxides (NMC), they theoretically have a higher specific energy. In addition to the standard redox activity of the transition metals, capacity is gained from the redox activity of oxygen, which is accessible by charging to high voltages. As a result, LMR is prone to transition metal dissolution, which can be deposited on the anode, increasing the risk of lithium plating in cells with graphite anodes. Due to the reactivity of metallic lithium, the deposited lithium on graphite is quickly consumed during the charging and discharging cycles. This not only increases safety risks, but also ensures that the capacity of the cells decreases rapidly. A research group from the Frontier Research Laboratory of LG Energy Solution in Münster – a cooperation of MEET Battery Research Center, Helmholtz Institute Münster of Forschungszentrum Jülich and the Advanced Cell Research Center of LG Energy Solution − has now investigated the influence of the voltage window on the electrochemical performance of cells with LMR cathodes and graphite anodes. The researchers identified an ideal voltage window in which cells with LMR cathodes achieve a competitive performance compared to cells with NMC cathodes.
Specific Cut-off Voltage During Formation and Aging Cycles
Previous research mostly concentrated on the electrochemical investigation of cells with an LMR cathode and a thick lithium metal anode. Their advantage: The high lithium content in the anode compensates the loss of active lithium and obscures the effects of dissolved transition metals. “The influence of the voltage window on the electrochemical performance of LMR cathodes is often neglected, but is an important factor, especially for cells with LMR cathodes and graphite anodes”, says Anindityo Arifiadi, scientist at MEET Battery Resreach Center and the Interational Graduate School BACCARA.
MEET researcher Dr Johannes Kasnatscheew adds: "The difficulty with LMR is that a high voltage is required for oxygen activation and thus the specific energy. At the same time, a sufficient lifetime requires a low voltage." The solution is to vary the voltage during formation and cyclization. The formation cycles should occur at a cut-off voltage of 4.5 volt (V). This is enough to activate the redox activity of oxygen and improves the cycling stability of the cells. During aging cycles, the voltage should remain between 4.3 and 1.9 V to achieve a stable cycling performance.
Arifiadi concludes: “Within this optimized voltage window, cell performance can be further stabilized via modifications such as coating, doping or electrolyte additives to enable practical application of LMR-based lithium-ion batteries.” The results should also establish standard voltage windows for LMR || graphite cells, which may help improve the comparability of future results of other research groups.
Entire Study Online Available
Detailed results of the study have been published by the authors Anindityo Arifiadi, Tobias Brake, Feleke Demelash, Bixian Ying, Dr Karin Kleiner, Dr Simon Wiemers-Meyer and Dr Johannes Kasnatscheew, MEET Battery Research Center, Prof. Dr Martin Winter, MEET Battery Research Center and Helmholtz Institute Münster, and Dr Hyuck Hur, Advanced Cell Research Center of LG Energy Solution, in the journal “Advanced Energy & Sustainability Research”.

