Potential Research Projects


Molecular Dynamics (MD) simulations are an efficient tool to study the structural and dynamic properties of battery related materials. However, the accuracy is often somewhat limited due to the use of empirical force fields. In recent years, machine learning approaches have been developed that allow the development of force fields derived directly from quantum mechanical information. In this way, it is possible to perform MD simulations with quantum mechanical accuracy.
This project aims to use MACE, which is the state-of-the-art machine learning approach [1]. It is based on the use of graph neural networks. We plan to apply MACE to simulate a variety of liquid electrolytes. Recently, transferable force fields based on MACE have been developed, e.g. for the most relevant elements of organic chemistry [2]. As a first benchmark, the results can be compared with previous simulations from our group using polarizable force fields. These simulations already gave excellent agreement of structural and dynamic properties with experiments [3]. Further improvements and a larger molecular diversity can be achieved by active learning methods involving additional quantum mechanical calculations. The results will be compared with experimental results obtained within BACCARA.
In summary, by exploiting MACE's strengths in efficient and accurate predictions, we aim to overcome current limitations in the simulation of electrolytes and open up new possibilities for computational studies in electrochemistry and materials science.
[1] I. Batatia et al., arXiv: 2206.07697.
[2] D.P.Kovacs et al., arXiv: 2312.15211.
[3] M. Maiti et al., Phys. Chem. Chem. Phys. 2023, 25, 20350-20364.
Contact Person:
This research project investigates the intricate relationship between potential energy landscapes and atomic-scale structure in solid-state materials, focusing specifically on ionic movement in alkali metal oxide systems. Building upon previous simulation studies of silica systems, we aim to elucidate the fundamental mechanisms governing ion transport in these materials, particularly below the glass transition temperature. The project centers on characterizing structural, energetic, and dynamical properties of alkali metal oxide systems, with particular emphasis on lithium ion dynamics. Through molecular simulations and theoretical modeling, we will investigate how individual lithium ions perform discrete jumps within the silica network structure, examining transitions between well-defined sites provided by the silica framework.
A key focus lies in developing and applying potential energy landscape (PEL) concepts to understand ion transport mechanisms. By analyzing the topology of local minima and their relationship to dynamical processes, we will take into account the underlying cooperativity. Comparison with NMR experiments can provide crucial validation of the simulation results. Through this integrated theoretical-experimental approach, we seek to advance our fundamental understanding of ionic transport mechanisms, ultimately contributing to improved functional materials for energy-related applications.
Contact Person:
Prof. Dr. Andras Heuer
andheuer@uni-muenster.de
Solid state batteries promise increased energy density whilst avoiding some of the safety concerns associated with current liquid electrolytes. In particular, the materials class of Na Super Ionic CONductors (NASICONs) has been proposed as solid electrolytes due to their potential to yield high ion conductivity and stability. In this project, ion transport and thermal conductivity in novel battery materials with the general stoichiometry Na1+xZr2SixP3-xO12 (x=0–3) will be studied using (ab initio) molecular dynamics. A particular focus lies on unravelling the dependence ion transport on local order, defects, and impurities or dopants. In addition, we will study the thermal properties of solid state electrolytes using equilibrium and nonequilibrium molecular dynamics techniques as they play an important role in battery performance. A particular goal is to disentangle electronic, phononic, and diffusive contributions to the thermal conductivity.
Initially ab initio simulations based on density functional theory (DFT) will be performed. To alleviate the associated computational limitations, a previously developed acceleration scheme will be employed. In addition, the data generated from the DFT simulations will be used to train neural network potentials which will allow simulations on significantly extended time and length scales.
Contact person:
Solid state batteries are an ideal option to use large capacity materials as active materials, while at the same time mitigating detrimental side reactions that stem from liquid electrolytes. However, a careful design of the microstructure and composition of the cathode composite, composed of solid electrolyte and the active material, is needed. In this doctoral project a deeper understanding of the underlying reaction and influence of microstructure on the behavior of solid state batteries needs to be elucidated, with the question of how can better design these composites. Within the scope of the project, you will learn how to synthesize sulfide based superionic conductors, form composite electrodes and measure the underlying transport properties using impedance spectroscopy that are then compared with cycling of solid state batteries.
Contact Person:
Sulfide or thiophosphate based solid ionic conductors currently typically achieve ionic conductivities between 1 and 10 mS/cm. These conductivities are sufficient to study the influences and reactions occurring in solid state batteries, but for high energy density cells much faster ionic transport is needed. In this doctoral project, pertinent materials will be investigated for their structure – transport correlations in order to better understand and push ionic conductivities in materials. Within the scope of the project, you will learn how to synthesize sulfide based superionic conductors, how to analyze their structure and ionic transport.
Contact Person:
Most battery cell components such as polymers and additives must be renewable and sustainable for the next generation of “green” batteries. Microbial polysaccharides and small molecules, so-called bio-additives, can be produced in bioreactors under controllable and reproducible conditions on a large scale, thus allowing economic and ecological production. The application of some plant-based and microbial polysaccharides as well as bio-additives as battery components has been evaluated, most of them showing a good performance. Based on a broad variety of microbial polysaccharides available at AG Schmid, the applicability of different native and engineered microbial polysaccharides will be evaluated for their suitability to function as polyelectrolytes/separators or binders for high-capacity active materials in rechargeable batteries. From various natural microbial polysaccharides, the most promising one will be selected and further engineered to increase stability and performance (acetylation, pyruvylation, carboxymethylation, etc.). As criteria for their suitability as battery components, the polysaccharides will be evaluated concerning their function in battery cells in terms of mechanical and electrochemical stability, ionic conductivity and wettability (polyelectrolytes), as well as processability and compatibility with active materials (binder). The polysaccharides will be characterized with state-of-the-art active materials (e.g., for lithium-ion cells) in terms of electrochemical performance to demonstrate the competitiveness of the “green” battery cells compared to classical ones. In addition their compatibility with further bio-additives such as green solvents will be evaluated.
Contact Persons:
One factor contributing to the poor cycling performance of lithium-ion batteries is the loss of active lithium from the anode surface, caused by parasitic side reactions during the formation of the solid electrolyte interphase (SEI). The SEI can be enhanced by adding suitable additives that accelerate the formation of a thin, stable, and flexible layer, resulting in reduced internal resistance, increased power capability, and a longer battery lifespan. Leveraging our expertise in organic chemistry and catalysis, our group focuses on the molecular design of film-forming additives, advancing the fundamental understanding of key substrate parameters to develop a more systematic approach to electrolyte additive design.
Contact Person:
The development of new electrolyte additives for batteries has so far been a rather random discovery process. While knowledge and logical insights into the mechanisms of these additives have accumulated over time, the development of newer and more effective additives has often relied on a certain degree of luck. This project aims to deepen the understanding of the structure-activity relationship by employing high-throughput experimentation (HTE) to identify connections between chemical structures and the corresponding mechanisms and performance improvements in the cell. With this data, we can gain a more detailed understanding, enabling a more rational approach to the design and synthesis of new additive structures. These new structures will have a significantly higher likelihood of addressing the specific challenges of different battery types.
Contact Person:
We will explore new concepts for self-healing in lithium ion batteries based on responsive polymer microcapsules. The capsules will be filled with liquid or solid agents that can repair damaged electrodes or regenerate the solid-electrode interface. The capsules are composed of polymers which are thermoresponsive due to cross-links which autodissociate at a threshold temperature. Since the capsule walls are rather thin, it is expected that the capsules are also sensitive to mechanical stress, i.e. cracks or strain or other types of physical damage. In the course of the project, we will pursue parallel approaches that will enable the encapsulation of either liquid or solid agents.
Contact person:
As part of the ongoing energy transition, renewable energy sources such as photovoltaic and wind power already account for approximately 50.5% of Germany’s electricity mix. However, the available capacity for energy buffering currently covers only about 5% of the actual demand. To fully harness the fluctuating output of renewables, innovative and scalable stationary energy storage technologies are essential.
Redox-flow batteries (RFBs) are widely regarded as a promising solution for large-scale, long-duration energy storage. This project focuses on the development of novel redox-active organic catholytes featuring high electrochemical stability, good solubility, and reversibility. The goal is to design and understand molecular structures that are optimally suited for use in aqueous and non-aqueous RFB systems.
In addition, a key innovation lies in the concept of solid boosters – solid-state additives that selectively enhance the redox capacity without adversely affecting viscosity or chemical stability. These hybrid approaches offer a pathway to significantly improve the energy density and cycling performance of RFBs, contributing to the development of sustainable, grid-scale energy storage solutions.
Within the scope of this project, you will explore new classes of organic redox-active molecules and investigate how solid booster components influence the overall electrochemical behavior. Emphasis will be placed on the fundamental correlations between molecular structure, redox mechanism, and long-term stability.
The project is embedded in the interdisciplinary research environment of the Helmholtz Institute Münster (HI MS), and involves close collaboration with leading groups in materials design, synthesis, and full-cell testing.
Contact Persons:
Current, straightforward approaches for the optimization of existing functional electrolytes often lead to solutions where specific properties can only be improved at the expense of other relevant ones, implying that current liquid electrolytes are already close to their optimum performance and that major gains can only be achieved with substantially altered formulations.
The majority of current research activities is focused on commercially available electrolyte components, limiting the possible knowledge gain of the underlying processes in the battery. To design innovative electrolytes for the next generation of batteries, one must leave the “comfort zone” of commercially established compounds and explore the larger uncharted molecular universe.
This work comprises design, tailored synthesis and comprehensive physicochemical, electrochemical, analytical and structural study of targeted molecules for advanced Li- and Na-based electrolytes (on electrolyte and lab cell level) and their effectiveness in resulting cell chemistries, highlighted by the structure-property-reactivity-performance-safety relationship. In addition, this approach enables to further tailor the vital properties of (multi)-functional electrolyte components for targeted application(s).
Contact persons:
Polymer-based lithium metal batteries are currently commercially exploited but require further improvements in view of fast charge application and energy density. Therefore, the design of novel polymer hybrid electrolytes and suitably tailored composite cathodes is necessary. In this doctoral project, you will be acquainted with the preparation and processing of polymer hybrid electrolytes and composite cathodes, also obtaining insights into charge carrier transport by combining impedance and NMR spectroscopy analysis. In this way, the reversibility of Li metal inventory as well as the evolution of resistances upon battery operation will be elucidated, thus allowing for further advancement of polymer hybrid electrolytes.
Contact person:
PD Dr. Gunther Brunklaus
g.brunklaus@fz-juelich.de
gbrunklaus@uni-muenster.de
Battery cell aging refers to the deterioration of battery properties during electrochemical cycling and storage. Numerous analytical methods are available to determine individual aging processes. Usually battery cells that show a pronounced aging behavior are analyzed in this regard. This can be due to the use of materials that are still in the development stage. In these cases, individual aging effects are usually easy to detect. The situation is different for already optimized battery cells that age only very slowly. It is likely that the aging effects of fast and slow aging cells differ not only quantitatively but also qualitatively.
The aim of this doctoral thesis is to examine slowly aging battery cells using a very wide range of analytical methods to find out what causes the aging that is still taking place. In this context, collaboration with other doctoral students will be very important, since not every method can or should be carried out by one person. Furthermore, it is important to show what is necessary from an analytical point of view to detect weakly pronounced aging effects. The development and optimization of further methods for this application will complete the doctoral thesis.
Contact Persons:
Prof. Dr. Martin Winter
martin.winter@uni-muenster.de
Dr. Sascha Nowak
sascha.nowak@uni-muenster.de
The properties of battery cells such as lithium ion batteries, lithium metal batteries or sodium ion batteries are largely determined by the internal chemical and electrochemical reactions. In addition to desired reactions such as the intercalation and deintercalation of cations, a large variety of undesired reactions occur which can impair the properties of the cells and contribute to accelerated aging of the cells. Reactions at the electrode-electrolyte interfaces, which can be influenced by the addition of electrolyte additives, have a particularly strong effect. Whether with or without electrolyte additives, the reactions are largely unexplained, which means that it is still unclear why some additives have a positive effect on battery properties.
The aim of the doctoral thesis is to elucidate reactions in battery cells, among other things with the help of isotope-enriched materials. Analytical devices that are suitable for this are TOF-SIMS and NMR spectroscopy.
Contact Persons:
The amount of mobile, thus between anode and cathode transferable, “active” lithium does determines the cell capacity and is regarded by the “mass balance” (which is actually a capacity balance) between the electrodes. Through electrochemical decomposition of the electrolyte and the resulting formation of lithium containing protective layers i.e., the solid electrolyte interphase (SEI) on the anode and the cathode electrolyte interphase (CEI) on the cathode, active lithium is irreversibly immobilized, which means that a part of active, transferable lithium is “lost” for the further charge/discharge reactions. The immobilization of lithium at the graphite anode is locally very heterogeneous Furthermore, due to structural and compositional changes at the cathode/electrolyte interface and the resulting metal dissolution of the cathode, the growth of the SEI on the anode is further promoted. Overall, this “aging” leads to an increased active lithium loss due to the formation, reformation and growth of the SEI and CEI during cycling, resulting in capacity fading and less available specific discharge energy of the battery cell. The same principle can be applied to next generation batteries like sodium ion batteries (SIBs).
Contact Persons:
Limited Li resources in combination with the rapidly increasing demand for energy storage devices makes more abundant metals than Li highly promising for “next-generation” batteries. Especially Na, K and Mg, close to Li in the periodic table, with a far higher terrestrial abundance, are of prime interest for the currently emerging beyond-Li technologies. A challenge lies in transferring characterization methods established for Li-based electrolytes to beyond-Li electrolytes. For example, for quantifying the transference number as a key figure for efficient ion transport, the common electrochemical experiments suffer from metal surfaces which are hard to control, while in alternative NMR transport experiments the corresponding nuclei are not detectable.
The Schönhoff lab in Physical Chemistry is experienced in NMR transport experiments, with which diffusion coefficients of electrolyte species are measured. Furthermore, based on ion velocity measurements, mobilities of electrolyte species are determined by electrophoretic NMR (eNMR). Recently, a method based on combining eNMR and impedance spectroscopy was demonstrated, termed eNMR/IS (https://doi.org/10.1021/jacs.3c12272), which allows to determine transference numbers even for not directly detectable metal ions. Exploiting these possibilities, this PhD project will study a wider range of beyond-Li systems, involving Na, K, Mg, as well as currently very promising Zn- and Al-based systems, in liquid as well as in polymer electrolytes. Ion transport mechanisms will be identified from combining NMR studies of ion transport with ion coordination experiments in order to identify specifically optimized electrolyte formulations.
Contact Person:
While high-concentration liquid electrolytes (HCEs) achieve great electrochemical performance advancements in metal-based batteries (such as Li and Na) compared to low concentrated analogues, they still suffer from high viscosity, limiting ionic conductivity. A novel approach are localized high-concentration electrolytes (LHCE), where a third component next to conducting salt and solvent, the diluent, is added to reduce the viscosity without participating in ion solvation. With this addition, a heterogeneous structure consisting of salt-rich and diluent-rich phases is created. Thereby, the local environment of the ions, which is responsible for the benefits of a HCE, remains unchanged and the main disadvantage, the low ionic conductivity, is improved.
This project will explore the implications of structural heterogeneity and interfacial interactions between conducting salt- and diluent-rich phases with the goal to develop optimized electrolytes for Li- and beyond Li ion batteries. To this end, the methods of three research groups are available in a collaborative effort: NMR experiments of ion transport and local dynamics (Schönhoff lab) will be combined with MD simulation analysis of local ion coordination environments as well as mesostructure analysis by molecular dynamics (MD) simulations (Diddens group). Finally, electrochemical and safety related characterization of resulting cell chemistries is planned in the Cekic-Laskovic group at HI MS. The student will obtain specific training with all these methods and use the possibilities of the three labs to identify molecular compositions which make optimal use of the concept of heterogeneity to develop high performance LHCE.
Contact Persons:
Prof. Dr. Monika Schönhoff
schoenho@uni-muenster.de
Dr. Diddo Diddens
d.diddens@fz-juelich.de
Dr. Isidora Cekic-Laskovic
i.cekic-laskovic@fz-juelich.de
With the combined requirement of high ion conductivity and high mechanical strength of metal ion conducting electrolytes, polymer gel electrolytes form a beneficial compromise between liquid electrolytes and salt-in-polymer electrolytes. They can be formed by combining a metal salt (e.g. Li, Na-based) and a liquid electrolyte (e.g. organic solvent or ionic liquid) with a polymer, where the latter can be covalently crosslinked, or in some cases the metal ions serve as a crosslinker by metal coordination. Various concepts based on different monomer structures (cationic, anionic, zwitterionic, metal coordinating, non-coordinating) are currently being pursued and have partly led to beneficial conductivities in spite of mechanical reinforcement. While most studies provide rather empirical optimization of the overall properties, the envisioned project will deliver in-depth molecular level insight into the mutual interactions of the different species in such complex electrolyte formulations. Spectroscopic methods such as NMR chemical shifts and Raman analysis will provide a thorough understanding of ion coordination and competing interactions of different species. Transport experiments by pulsed field gradient NMR methods will demonstrate the consequences of tuning mutual interactions of different species by involving varying structural motifs, e.g. of the polymer building blocks. The detailed knowledge gained from this approach will concern pair and cluster formation, crosslinking effects, competing interactions of different species to the metal cation, and it will finally aid the identification of optimized chemistries of polymers for polymer gel electrolytes.
Contact Person:
While the lithium-ion technology is already mature, significant research is still being done to optimize sodium-ion batteries and to develop solid-state batteries. A deeper understanding of electrochemical mechanisms and structure-property relationships is crucial for a target-oriented optimization of functional anode, solid electrolyte, and cathode materials. In this doctoral project, novel battery materials will be investigated with respect to their structure, ionic transport processes and behavior during electrochemical cycling, using ex situ and operando NMR techniques as the main methods. Within the scope of this project, you will learn to synthesize battery materials, test their electrochemical performance, investigate their microscopic ionic transport and evaluate their function in battery cells outside and inside an NMR spectrometer.
Contact Person:
Within this PhD thesis, laser- and mass spectrometry-based analytical methods will be developed to identify and localize side products of electrochemical conversions on cathode and anode surfaces of lithium ion batteries (LIBs). As the solid-electrolyte interface (SEI) and the cathode-electrolyte interface (CEI) play important roles for the performance and the lifetime of LIBs, the battery properties can be better understood and thus optimized based on an excellent knowledge of the surface reactions.
Laser desorption/ionization using a laser wavelength of 355 nm will be selected to transfer materials into a time of flight-mass spectrometer (ToF-MS), which allows to record accurate m/z ratios of the analytes. A trapped ion mobility spectrometry (TIMS) device provides additional selectivity to address these complex samples. To enlarge the range of applicable analytes, laser ablation with atmospheric pressure-post ionization mass spectrometry (LA-API-MS) based on dielectric barrier discharge ionization (DBDI) and atmospheric pressure chemical ionization (APCI) will be used for analytes with limited polarity.
Software tools as MZmine3 for integrative analysis of multimodal mass spectral data and the molecular networking platform GNPS will be used for data annotation and identification. The dimension reduction technique UMAP shall serve as another advanced computational tool for improved data interpretation.
Contact person:
The topic of this PhD thesis is the development and application of an automated analytical platform for speciation analysis in battery electrolytes using ion chromatography (IC) coupled to inductively coupled plasma-optical emission spectrometry (ICP-OES) and inductively coupled plasma-mass spectrometry (ICP-MS).
Background of this thesis is the need for an improved understanding of the composition of battery electrolytes, which may contain complex mixtures of reaction and decomposition products of the electrolyte constituents upon electrochemical degradation, hydrolysis and other stressors. Reaction products are separated by ion chromatography on an anion exchange column and detected by ICP-OES or ICP-MS, respectively, depending on the analyte concentration. Depending on the nature of the electrolytes, phosphorus, sulfur, boron and chlorine species may be formed, which are quantified by ICP-MS based on external and internal calibration strategies. A respective automated sample injection, dilution and calibration system shall be established based on this work.
Unknown reaction products shall be identified using hydrophilic interaction liquid chromatography (HILIC) with electrospray mass spectrometric (ESI-MS) detection. Modern software tools shall be applied to assist the unambiguous identification of the formed reaction products.
Contact person:
Sodium Ion batteries are promising alternatives to state-of-the-art Li ion batteries, as, among others, the Na abundance can relevantly decrease costs, though is compromised by lower gravimetric/volumetric energy. Aim of this study is to unravel the bottlenecks and diagnose the failure mechanisms at harsh conditions, i.e., fast charge, high voltage and/or application at wide temperature range; with the purpose to systematically improve and design the active/inactive materials and conditions.
Contact persons:
Prof. Dr. Martin Winter
martin.winter@uni-muenster.de
Dr. Johannes Kasnatscheew
johannes.kasnatscheew@uni-muenster.de
The anodes in Li ion batteries (LIBs), as state-of-the-art, are based on graphite active materials. Though, they have relatively high specific capacities compared to the cathodes, their low electrode density leads to high electrode volume and relevantly limits the energy density of the LIB. The Si-based active material is an abundant and promising alternative to graphite, though suffers from material stress-reasoned poor cycle life in course of pronounced volume expansion during charge/discharge cycling. This work aims to unravel the failure mechanisms on material level as well as the complex interplay between active and inactive materials within the composite electrode, as basis for systematic improvement- and material design strategies.
Contact persons:
Prof. Dr. Martin Winter
martin.winter@uni-muenster.de
Dr. Johannes Kasnatscheew
johannes.kasnatscheew@uni-muenster.de
A major advantage of zinc batteries is the possibility of using aqueous electrolytes. However, the leaching of transition metals from the active materials on the cathode side is a major challenge that has not yet been solved satisfactorily. In this project, active materials are to be synthesized and the effect of the microstructure of the particles on leaching and cell performance will be investigated. Subsequently, the effects of different coatings can then be investigated on the optimized particles. In addition to active material particle synthesis and conventional electrochemical analysis methods (CV, EIS), the project will focus on SEM, XRD and AFM-based measurement techniques.
Contact persons:
Prof. Dr. Martin Winter
martin.winter@uni-muenster.de
Dr. Kerstin Neuhaus
k.neuhaus@fz-juelich.de
The concept of “water-in-salt” electrolytes (WiSE), such as lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) in aqueous solution, was introduced to overcome the electrochemical stability window limitations of conventional aqueous electrolytes, enabling an extended stability window of over 2 V. In WiSE systems, anion solvation and the local structure as well as binding of water differ significantly from those in traditional salt-in-water electrolytes. At electrode/electrolyte interfaces, the molecular arrangement plays a key role in expanding the electrochemical stability of WiSE and in forming a solid-electrolyte interphase (SEI)—both of which remain largely underexplored for WiSE. This project will apply vibrational sum-frequency generation (SFG) spectroscopy to investigate, in situ, the molecular structure at the electrode–electrolyte interface as a function of electrode potential and electrolyte composition. SFG spectroscopy is a nonlinear optical technique that is inherently interface-selective and can probe vibrational resonances of surface-adsorbed molecules, enabling detailed insights into interfacial molecular structures which we aim to modify with the electrode potential and the composition of the bulk electrolyte.
Contact person:
Lithium (Li) metal has become the focus of extensive research in the past years. Its high gravimetric capacity of 3860 mA h g−1 and low standard reduction potential of −3.04 V vs. Standard hydrogen electrode (SHE) make it an ideal choice for a negative electrode active material in high energy density batteries. Nonetheless, the application of Li metal has major challenges. The decomposition of the electrolyte, the formation of the Solid Electrolyte Interphase (SEI) and the accumulation of inhomogeneous Li metal deposits, respectively ‘high surface area lithium (HSAL)’ and ‘dead Li’ cannot only result in active lithium loss (ALL) but also safety concerns. In particular, the risk of short-circuits by HSAL and the high reactivity of liquid ammable electrolytes with HSAL can be hazardous. To overcome these safety concerns, solid electrolytes (SE) and electrolytes based on ionic liquids are considered as safer alternatives.
A soild electrolyte (SE), which has been invented in MEET/HI MS will be used in a NMC cell. The build-up of the electrolyte can be seen in figure 1. The goal of the work will be to incoorperate the SE in the cathode material and the optimization of the SE. The work includes also a collaboration with the US and Japan.
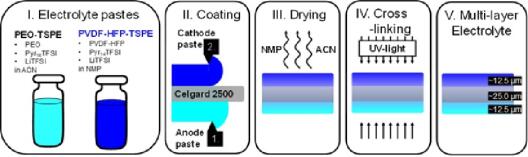
Figure 1: Build-up of a soild electrolyte.
Herbers, L. et al., Adv Energy and Sustain Res 2023, 4.
Zhang, M. et al., Journal of The Electrochemical Society 2019, 166, A2142-A2150.
Contact persons:
The overall goal is to identify new, innovative materials which show on the one hand optimal technical properties and on the other hand low human and environmental toxicity. Toxic effects of selected state-of-the-art additives have been reported. In a prior PhD-theses mutagenic and genotoxic potential were identified as one important AOP. Nevertheless, for human risk evaluation it is important to understand the respective toxic behaviour after cell cycling – model for aged batteries. We showed that the pure electrolyte becomes less toxic during cycling. Dependent on the used additives the toxic potency didn’t change over different life time stages. Overall, to close the knowledge gap about their environmental behaviour, different toxicity test systems will be used to investigate the environmental compatibility of new battery materials. Established test systems will be applied to test materials which will be provided by the synthetic groups within the BACCARA consortium.
The Institute of Food Chemistry provide a deep knowledge about analysis of toxic food compounds / contaminants. Cell-based test systems are routinely used to determine cytotoxicity, genotoxicity and mutagenicity. Furthermore, the impact of food toxicants or environmental chemicals on humans is studied based on high-performance liquid chromatography- mass spectrometry techniques.
The candidates should hold a Master or equivalent degree in Food Chemistry, Toxicology or (Bio-) Chemistry. Experiences with analysis techniques (chromatographic separation techniques, mass spectrometry) are required. Basic skills in cell culture and toxicity tests are an advantage.
Contact person:
Prof. Dr. Melanie Esselen
esselen@uni-muenster.de
Prof. Dr. Hans-Ulrich Humpf
humpf@uni-muenster.de
The rapid evolution of battery technologies is pivotal for addressing the increasing demand for energy storage solutions, particularly in the context of renewable energy integration and electric vehicles. A techno-economic assessment (TEA) in combination with a life cyle assessment (LCA) of these novel battery technologies entails a comprehensive analysis and evaluation of their technical performance, scalability, economic viability, and environmental impact.
In this PhD project, one or more next-generation battery storage technologies will be holistically investigated and analyzed. The assessment will begin with a review of the current state of the art, including proposed active materials, cell design, production pathways and postulated advantages as well as challenges. The following in-depth assessment is divided into three main areas:
(a) Technical performance evaluation and prediction
Assessing the battery technology's key performance indicators, such as energy density, cycle life, charge/discharge rates, and safety features;
(b) Economic and scalability evaluation and prediction
Analyzing cell production costs, including raw materials, active material synthesis, manufacturing processes, and supply chain logistics. This will also involve evaluating scalability, and market competition to determine economic viability and a suitable market;
(c) Life cycle and sustainability assessment
Conducting a lifecycle analysis to evaluate the cradle-to-grave environmental impacts, of the technology, among others the carbon footprint, from raw material extraction to disposal or recycling.
This holistic assessment will provide stakeholders, including manufacturers, investors, and policymakers, with the insights necessary to make informed decisions regarding the adoption and commercialization of these battery technologies.
Contact Person:
Queensland is a frontrunner in residential solar energy adoption, with approximately 33% of households connected to Energex and Ergon Energy Networks having installed solar photovoltaic (PV) systems by the end of 2022. However, the adoption of battery energy storage systems (BESS) remains remarkably low, at only 1.7%.
This discrepancy presents significant challenges for both economic and technical efficiency, as excess solar energy cannot be effectively stored or redistributed, thereby limiting the potential of Virtual Power Plants (VPPs). Understanding the structural and systemic barriers to battery adoption is therefore essential for unlocking Queensland’s renewable energy potential.
In contrast, Germany has successfully implemented various policies that have resulted in significantly higher adoption rates of residential battery systems, offering valuable insights for Queensland. However, Germany faces its own challenges due to a strong dependence on wind energy.
This project aims to explore the structural, economic, and infrastructural barriers to residential battery adoption through a comprehensive techno-economic assessment (TEA) and life cycle assessment (LCA) of existing energy storage solutions as well as potential next-generation technologies. It will investigate key structural factors, the role of information systems, systemic conditions affecting VPP participation, and valuable lessons from Germany’s experience. Utilizing a mixed-methods design, the study will involve data analysis, system and cost modeling, life cycle assessments, and potentially qualitative interviews, quantitative surveys, and system behavior modeling, culminating in evaluations of technical performance and environmental impacts.
The project aims to generate actionable insights for designing informed tools that facilitate battery adoption and VPP engagement, contributing to cross-site collaboration between Australia and Germany and potentially fostering technology exchange.
Contact Person:
Prof. Dr. Simon Lux
simon.lux@uni-muenster.de
Summary: The transformation of the automotive industry toward electrification is leading to a rapid rise in the demand for batteries. However, once batteries used in electric vehicles have reached the end of their lifespan, the question arises how to handle the sheer volume of battery waste. While the environmental benefits of recycling batteries are clear, the economic feasibility of lithium-ion battery recycling is less straightforward. The aim of this interdisciplinary research is to address this issue by exploring sustainable business models for different battery recycling methods, with a particular emphasis on the concept of circular economy. The topic of the project sits squarely at the intersection of battery chemistry and innovation management.
Your profile: You have completed or are about to complete a master degree in business chemistry, chemistry or chemical engineering. A good understanding of battery chemistry as well as principles of management (e.g., acquired in technology and innovation modules or industry internships) are essential. Candidates are expected be passionate about interdisciplinary research and engagement with industry.
Contact person:
Prof. Dr. Stephan von Delft
stephan.vondelft@uni-muenster.de
Summary: Recycling of end-of-life electric vehicle (EV) batteries and their reuse (2nd use) in stationary energy storage both play an important role in creating a circular battery economy. While recycling and 2nd use are important imperatives in the circular economy, they may be in conflict with one another. The aim of this research project is to compare the two alternatives (recycling and 2nd use) by modelling material flows and climate effects of end-of-life EV battery supply scenarios and the effect of recycling and 2nd use on battery demand and saved greenhouse gas emissions in Europe. Results of this research should support policymakers and industry in their decision about which option is more beneficial.
Your profile: You have completed or are about to complete a master degree in business chemistry, chemistry or chemical engineering. A good understanding of battery chemistry and an interest in circular economy are essential. Candidates are expected be passionate about interdisciplinary research and engagement with industry.
Contact person:
Prof. Dr. Stephan von Delft
stephan.vondelft@uni-muenster.de
The environmental footprint of a battery, as well as the total cost of ownership, is highly dependent on the lifetime of the battery. Battery development for higher energy density and lower cost is driving rapid changes in cell design and materials. In order to control the impact of these developments on lifetime, methods are needed that allow rapid estimation.
The lifetime of a battery is highly dependent on operating conditions such as state of charge, current, depth of discharge, temperature and pressure. All these operating conditions influence the aging mechanisms that occur, e.g. Lithium plating, SEI dissolution/cracking, transition metal dissolution, electrical contact loss, electrolyte degradation. In addition, these aging mechanisms interact with each other. This leads to so-called path dependency in battery aging, meaning that a battery will age differently under certain operating conditions depending on the operating conditions to which it was previously exposed.
Working on this topic in the Cell System Group of MEET Battery Research Center, you will acquire a sophisticated knowledge of the chemical and electrochemical processes within the battery. You will prepare different battery cell designs, set up different electrochemical test procedures, and perform in-depth data and post-mortem analysis to identify and quantify the interactions of aging mechanisms. This will lead to an improved method for estimating battery lifetime and a better understanding of the chemical processes that reduce lifetime, allowing for lifetime improvement.
Contact persons:
Prof. Dr. Martin Winter
martin.winter@uni-muenster.de
Dr. Philip Niehoff
Philip.Niehoff@uni-muenster.de
Several materials and processes in lithium-ion battery production are sensitive to moisture. As a result, several drying steps are required during production, as well as the execution of several process steps in a dry room atmosphere. These steps are energy intensive and therefore increase costs and environmental impact.
Working on this topic in the Cell Systems Group of the MEET Battery Research Center, you will study the impact of different amounts of moisture in the electrolyte over time at the material and cell level on performance, as well as the underlying aging mechanisms. You will develop quality control methods to verify that no detrimental amount of moisture contact was present. You will prepare different reference experiments at material level, different battery cell designs, perform in-depth data and post-mortem analysis to identify and quantify the aging mechanisms that occur. This will lead to a better understanding of the chemical processes that degrade battery performance, allowing for lifetime improvement and an optimized manufacturing process that reduces cost and environmental impact.
Contact persons:
Increasing demands for energy storage systems have led from lithium ion batteries to the development of lithium metal batteries. Current advances towards thin-film lithium metal anodes or even the absence of a reservoir in lithium metal batteries offer potential for further improvements in energy density, specific energy, and cost reductions. However, commercialization is hampered by safety issues and insufficient cycle life.
Two factors are considered key for future high-energy LMBs. One of them is the reduction of the amount of lithium metal within the cell. While zero excess would implicate that irreversible lithium loss leads to direct and continuous capacity decrease, a minimized excess could greatly enhance the cells life time. Beyond that, inhomogeneous Li deposition and formation of detached “dead” lithium needs to be mitigated for successful commercialization of LMBs. Therein, structured substrates and functionalized surfaces are considered key. Therefore, different structures will be prepared and investigated combined with tailored coatings and electrolyte variations. Apart from that, the scalability and safety of the developed LMB concepts will be investigated in cooperation with the in-house pilot batteryline and the battery safety lab operated by the Cell Systems group of the MEET Battery Research Center.
Contact persons:
State-of-the-art production of positive electrodes involves fluorinated PVdF binder processed in critical solvents which represent a potential danger to operators and the environment. Thus, to enhance sustainability in electrode and battery cell production, the replacement of these substances is considered a crucial step. Beyond that, the imminent ban of fluorinated binders in the EU require direct action in research and development.
Working on this topic in the Cell Systems group at MEET Battery Research Center, you will evaluate different approaches for sustainable electrode production. Therein, the state-of-the-art processing will be compared with aqueous and dry processing with F-free binder systems. Especially for dry processing, the replacement of PTFE represents a huge challenge, which requires combinational approaches involving conductive additives and binder systems. IN case of aqueous processing, the high reactivity of active materials towards water need to be mitigated by processing additives that potentially even improve the electrochemical performance. You will be developing and evaluating the approaches with regard to sustainability, performance, scalability, and costs. Finally, the upscaling will be performed in cooperation with the in-house pilot batteryline.
Contact persons:
This research proposal aims to investigate a strongly decentralized battery value chain initiative inspired by the Airbus manufacturing model in Europe and to compare it with the traditional centralized gigafactory approach. In the Airbus model, specialized factories across Europe focus on specific segments of the value chain, while large airplane parts are transported throughout the continent. Similarly, in lithium-ion battery manufacturing, this would mean that electrode manufacturing, cell assembly, and cell formation occur in different factories, whereas in a traditional setup, all these processes take place in the same facility.
Therefore, as Europe transitions to electric mobility and renewable energy storage, understanding the dynamics of these two different models is crucial for developing an efficient and sustainable battery supply chain for Europe and the transition to a fully sustainable energy supply.
The main objectives of this research include the following:
Ecosystem Modeling: A simulation model will be developed to represent the interactions within the decentralized battery value chain. This model will consider various factors, including production capacity, supply chain logistics, and regulatory compliance.
Cost Assessment: Adetailed cost analysis, examining factors like capital expenditures, operational costs, and logistics will be performed, including the influence of subsidies or incentives from European policies.
Policy & Warranty Analysis: The research will involve a thorough examination of relevant European Union policies and regulations that affect battery production and sustainability, including initiatives like the European Green Deal and Circular Economy strategies.
Analyze Sustainability: A comprehensive evaluation of the environmental impact of both production models will be conducted. This will focus on aspects such as manufacturing emissions, production quality and end-of-life battery management.
Comparative Analysis: The decentralized and centralized models will be benchmarked against similar supply chains, such as the semiconductor sector, to highlight best practices and identify potential challenges.
The methodology for this research will encompass several steps:
A literature review to analyze existing research and case studies related to battery supply chains, sustainability, and policy frameworks.
Data collection from industry reports, expert interviews, and case studies to gather both quantitative and qualitative information.
Model development utilizing system dynamics or agent-based modeling to simulate the proposed battery ecosystem.
Establishing a comparison framework with key performance indicators (KPIs) to assess the different supply chain models.
The expected outcomes of this research include a comprehensive understanding of the trade-offs between decentralized and centralized battery production, identification of policy recommendations that support sustainable battery supply chains, and the development of a simulation model that stakeholders can use to evaluate various scenarios and make informed decisions.
In conclusion, this research will provide valuable insights into the future of battery production in Europe, contributing to the broader discourse on sustainable manufacturing practices and policy frameworks. By comparing innovative decentralized approaches with traditional centralized models, this study aims to guide industry stakeholders and policymakers towards more sustainable energy solutions.
Contact person:
Prof. Dr. Simon Lux
simon.lux@uni-muenster.de