PROJECT B11
Remodeling of Tricellular Junctions Controls Paracellular Transport
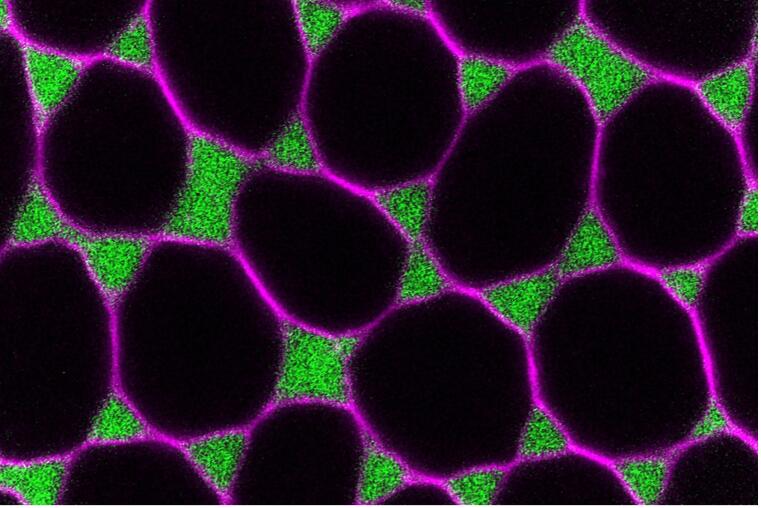
Cell-cell junctions in epithelia are dynamically remodeled to modulate the transport of liquids, ions and solutes, or to enable passage of migrating cells. During oogenesis in oviparous animals, tricellular junctions in the follicular epithelium open transiently to allow passage of yolk proteins for uptake by the oocyte. We study this process, referred to as patency, using Drosophila as a model system to understand how the dynamic disassembly and reassembly of adhesion complexes at tricellular junctions regulates epithelial permeability. Patency is initiated by the sequential removal of adhesion proteins (E-Cad, NCAM/Fasciclin 2, Sidekick) from follicle cell vertices and is terminated by the assembly of septate junctions that establish a tight epithelial barrier. Modulation of E-Cad levels regulates the extent of vertex opening, and patency is prevented by artificially stabilizing adherens junctions, by blocking endocytosis, or by increasing actomyosin contractility. We use live imaging, pharmacological and optogenetic approaches to investigate the mechanisms that mediate the opening of follicle cell vertices through modulation of actomyosin contractility and through local removal of adhesion proteins from the interface between three cells.


