PROJECT B05
Localized FGF-Receptor Signaling Controls Differentiation of Wrapping Glial Cells
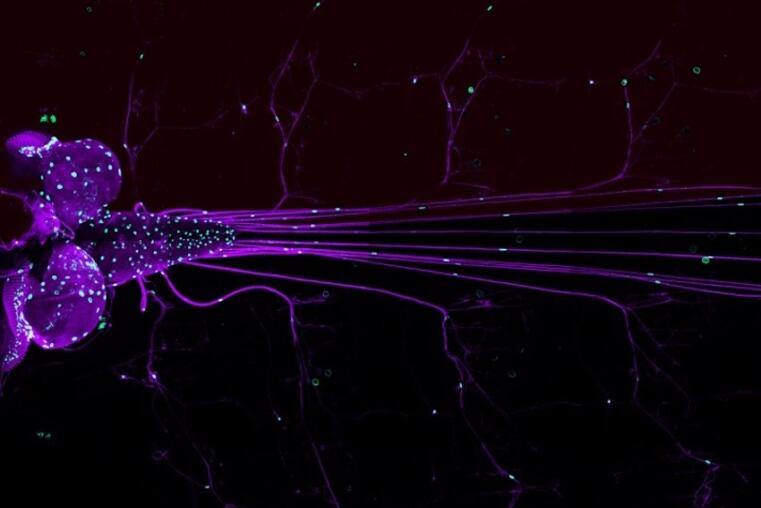
Efficient neuronal conductance requires insulation of axons by glial cell processes. Invertebrates, such as the fly Drosophila melanogaster, show a relatively simple extension of glial membranes around the axons, resembling Remak fibers formed by Schwann cells in the mammalian peripheral nervous system. In the framework of this CRC we will unravel the molecular pathways underlying glial differentiation at the interface of the axonal membranes in the Drosophila larva. Here, wrapping glial cells first form thin processes along axon fascicles to then ensheath the entire axon bundle. This differentiation process is controlled by fibroblast growth factor (FGF)-signaling. In the developing eye, the FGF-receptor is expressed at the neuron-glia interface along the entire length of the photoreceptor axon fascicle and is activated by FGF secreted by the axon. This suggests that localized activity of the FGF-receptor is important for triggering glial membrane growth around the axonal fascicle. Preliminary experiments identified several proteins required for normal differentiation of the wrapping glia, providing an entry point to dissect the molecular mechanisms that direct glial growth around the axon.


