Novel Electrolyte Composition Increases Lifetime of Silicon-based Anodes
In order to improve the performance of lithium-ion batteries, current research is attempting to further increase the energy density of the cells. The coupling of nickel-rich layered oxide cathodes and silicon-based anodes (e.g. SiOx graphite) with a simultaneous increase in the upper limit voltage above 4.3 V is considered to be a promising approach. At present, however, the instability of the electrolyte in lithium-ion cells with SiOx-based anodes still poses a major challenge, as it can lead to abrupt cell failure. A team from MEET Battery Research Center at the University of Münster and the Helmholtz Institute Münster (IMD-4; HI MS) of Forschungszentrum Jülich has now succeeded in achieving a significantly improved lifetime for lithium-ion batteries with silicon-based anodes by combining two electrolyte additives.

Interaction of the Additives Ensures Effective Protection of the Anode
At voltages above 4.2 V, transition metal dissolution of nickel, manganese and cobalt occurs in the cathode. The metals dissolved in the electrolyte migrate to the anode, where they lead to harmful reactions and lithium metal deposition. As a result, the energy of the battery drops sharply. The scientists have now developed an electrolyte in which the two additives 1,1′-sulfonyl diimidazole (SD) and fluoroethylene carbonate (FEC) are combined in a specific ratio. The interaction of the two substances forms a protective layer that significantly reduces the energy loss of the battery over the lifetime of the cell. “The anode is thus protected and survives the strong volume changes of the silicon largely unscathed,” MEET researcher Dr. Philip Niehoff explains the mode of action.
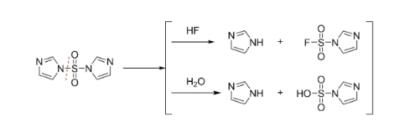
In addition, the remaining 1,1′-sulfonyldiimidazole suppresses transition metal dissolution by intercepting the harmful hydrofluoric acid produced by a reaction between the lithium hexafluorophosphate in the electrolyte and the residual moisture in the cells. “The novel additive combination stabilizes the solid electrolyte interphase (SEI) and protects it from attacks by this acid,” says Niehoff. Thanks to the improved lifetime and performance of the cells, the research results make an important contribution to the necessary cost reductions in the production of battery systems.
Detailed Results Available Online
The entire study has been published by the authors Feleke Demelash, Anindityo Arifiadi, Bastian Heidrich, Egy Adhitama, Christian-Timo Lechtenfeld, Niklas M. Abke, Dr Philip Niehoff and Dr Simon Wiemers-Meyer, MEET Battery Research Center as well as Matthias Weiling, Jian Fen Wang, Diddo Diddens and Masoud Baghernejad, Helmholtz Institute Münster (IMD-4; HI MS) of Forschungszentrum Jülich and Prof. Dr Martin Winter, MEET Battery Research Center and Helmholtz Institute Münster in the journal „Energy Storage Materials”.

