Follow-up Study Identifies Influence of Different Activators and their Mixing Approach on the Graphitization of Coffee Ground
Low cost and environmentally friendly production of graphite anodes from naturally available biomass resources such as coffee ground is of great importance to satisfy the increasing material demand for lithium-ion batteries. In a previous study, a team from MEET Battery Research Center at the University of Münster, the University of Seville, and Helmholtz Institute Münster of Forschungszentrum Jülich has already investigated the influence of the activator iron(III) chloride hexahydrate on the graphitization process of coffee ground, which only yielded limited development of the graphitic structure. Based on these results, the scientists have now analyzed four different iron-based activators. They paid particular attention to how a wet or a dry mixing approach to incorporate the activators favors the development of the graphitic structure and thus the performance of the battery cell.
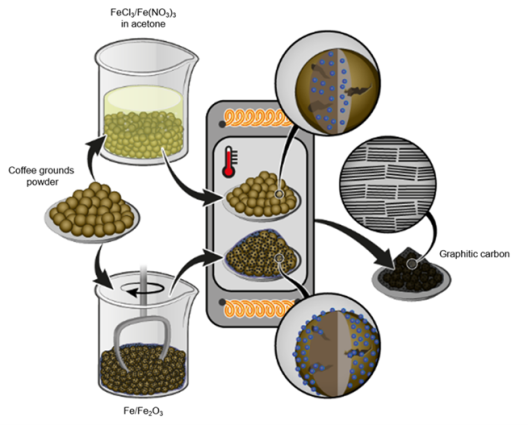
Physical Mixing Process Improves Electrochemical Properties
A possible, sustainable precursor material for the production of synthetic graphite is the biomass product coffee ground. In a study conducted in 2022, the scientific team of the involved research institutions discovered for the first time a saturation point for the degree of graphitization when using iron chloride as activator and coffee ground. The saturation point was achieved when the activator accounted for 40 percent of the total weight by mass and was heat treated at 2,000 degrees Celsius. Due to the volatility of the iron source used, various less volatile activators were of great interest in terms of their properties for the graphitization process. Therefore, the researchers compared the influence of four different activators: iron (III) chloride, iron (III) nitrate, iron (III) oxide and pure iron. They added the activator either in liquid form as an iron salt dissolved in an organic solvent or directly in solid form. “The direct physical addition of micrometer-sized iron particles improved the electrochemical properties of the coffee ground derived graphite the most,” says MEET scientist Lars Frankenstein. “Contrary to expectations, liquid impregnation prior to graphitization does not lead to a more satisfactory graphitization despite a generally initial more homogeneous iron distribution in the coffee ground.” This provides both cost advantages, since pure iron is cheaper than the iron solution, and time advantages in the manufacturing process: While the dry mixing approach takes only a few minutes, the liquid impregnation process takes several hours.
Further, the iron in the solution deposits as nanoparticles on the surface within the pores. Frankenstein classifies: “Larger particles of the powdered activator seem to be more beneficial to the graphitization of biomass than nano-structured activators. This indicates that the particle size might play a critical role on the graphitization mechanism. This phenomenon requires further research now.”
Complete Study Online Available
The detailed results of their study have been published by the authors Lars Frankenstein, Pascal Glomb, Dr Aurora Gomez-Martin and Dr Tobias Placke, MEET Battery Research Center, Prof. Dr Joaquin Ramirez-Rico, Departamento Física de la Materia Condensada and Instituto de Ciencia de Materiales de Sevilla, Universidad de Sevilla, and Prof. Dr Martin Winter, MEET Battery Research Center and Helmholtz Institute Münster, in the journal “ChemElectroChem”.

