Research Team Investigates Influence of the Electrolyte in Polymer-based Dual-ion Batteries
A promising addition to the lithium-ion battery (LIB) is the dual-ion battery (DIB). One of its advantages is that a variety of new and sustainable materials can be used. For example, expensive metals such as nickel or cobalt can be avoided. It also offers a high performance, which determines the fast-charging capability of the cells. The influence of the electrolyte on the performance of DIBs has not yet been sufficiently researched. A scientific team from MEET Battery Research Center at the University of Münster and the Institute of Organic and Macromolecular Chemistry at the Friedrich Schiller University Jena addressed this topic in a recent study. The researchers investigated the influence of different electrolyte formulations on DIBs with polymer-based cathodes.
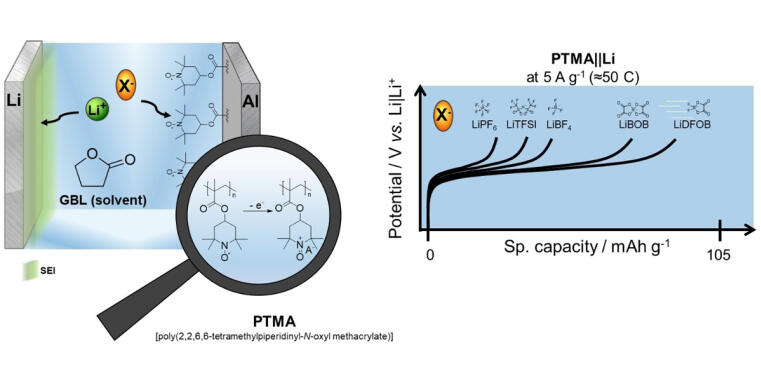
Fast-charging Capability Significantly Improved
In their study, the team used various electrolyte formulations based on lithium salts, which were dissolved in lactone-based solvents. “Lithium difluroro(oxalato)borate (LiDFOB) achieved the most promising results,” says MEET scientist Katharina Rudolf. The compound delivered up to 90 percent of its capacity at a rate of 50C. This corresponds to a charge and discharge time of 1.2 minutes. “This exceeds the capabilities of the commercial lithium-ion battery considerably in terms of the fast-charging capability. The electrolyte also improves the cycle life of the cells,” explains Rudolf. However, the LIB still has a clear advantage regarding energy density and self-discharge.
Another important finding of the researchers is that the determined performance of the DIB with the electrolytes investigated does not correlate solely with the ionic conductivity or anion size. “The functionality of the electrolyte in dual-ion batteries is therefore even more complex than previously assumed,” adds Rudolf. Further research is now needed to systematically develop the technology further, but also to understand the general principles of the electrolyte function in energy storage systems in more detail.
Entire Study Online Available
The detailed results of the study have been published by the authors Katharina Rudolf, Linus Voigt, Lars Frankenstein, Justin Landsmann, Dr Tobias Placke und Dr Johannes Kasnatscheew, MEET Battery Research Center, Prof. Dr Martin Winter, MEET Battery Research Center and Helmholtz Institute Münster, as well as Simon Muench and Prof. Dr Ulrich S. Schubert, Institute of Organic Chemistry and Macromolecular Chemistry at the Friedrich Schiller University Jena, in the journal “ChemSusChem”. The project is funded by the German Research Foundation.

