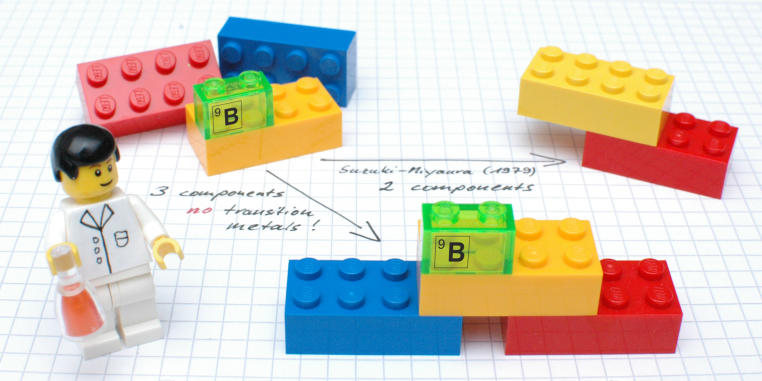





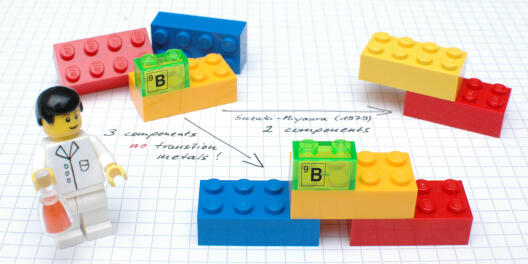
One of the greatest challenges for organic chemists is to create specific bonds between carbon atoms in various chemical components. This is, however, essential for the construction of complex, pharmaceutically active and biologically relevant molecules. The tools which are particularly important for this are so-called cross-coupling reactions, including the "Suzuki-Miyaura coupling, which was awarded the Nobel Prize for Chemistry in 2010. This reaction, used by the chemical industry in tonne scale, makes it possible to link two chemical components, although one of the components has to contain a reactive boron moiety. What is decisive for the reaction process is the presence of expensive transition metals such as palladium, which brings the two reactants together, so that in the end a carbon-carbon bond is formed.
In "Science" magazine, the group of Prof. Armido Studer presents a new approach which for the first time enables three – and not, as previously, two – chemical components to be "coupled" in one single reaction, without any metals to aid the process. The researchers succeeded in producing not only pharmaceutically relevant compounds containing fluorine, but also various γ-lactones. These organic compounds occur widely in various types of fruit and also, for example, as flavouring substances in whisky and cognac.The method includes the formation of two carbon-carbon bonds. "Unlike classic cross-couplings, however, the valuable boron moiety remains in the product," Studer explains. "At this point, further changes can then be made to the molecules in the same reaction vessel." This method makes it possible, he adds, to produce a large number of different derivatives.
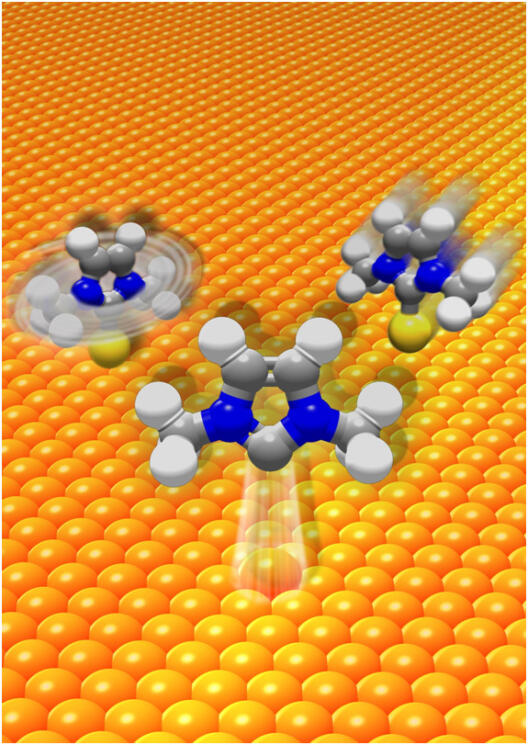
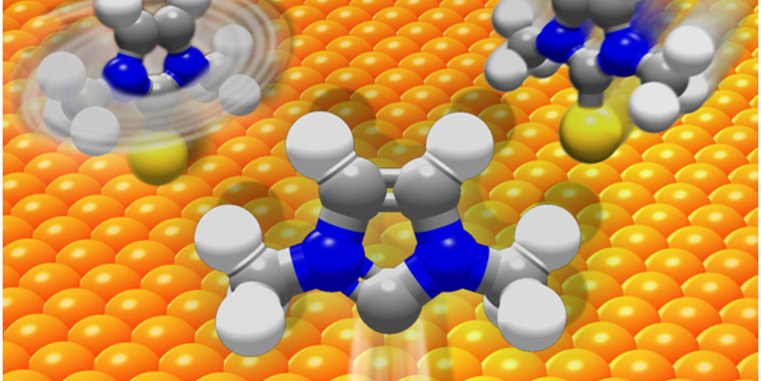
Self-assembled monolayers are molecules anchored to a surface. These molecules have been used to advance the fields of molecular sensors and electronics. Monolayers on gold surfaces are typically anchored by a thiol. One alternative to thiols are N-heterocyclic carbenes (NHCs). First isolated in 1991, NHCs have proved an important molecule for practical uses because it can serve as a ligand for transition metals as well as some main group elements. NHCs have also been used for organocatalysis, cross-coupling reactions, and pharmaceuticals. Research groups from Westfälische Wilhelms-Universität in Münster led by Professors Harald Fuchs and Frank Glorius, used scanning-tunneling microscopy analyses combined with first principles calculations of representative NHC monolayers on gold surfaces to determine how the monolayers can be both chemically robust and highly mobile. They found that the NHC bound to gold forms an adatom that then traverses the gold surface in a ballbot-type motion. Their report appeared in a recent issue of Nature Chemistry.
This study provides important insights into how NHC-Au monolayers form and behave. They seem to form a stronger bond than thiols with gold, but they also display mobility that is influence by the identity of the nitrogen substituents. The NHCs that tend to have weaker pi interacts with the gold surface readily forming adatoms that then move across the surface like a ballbot.

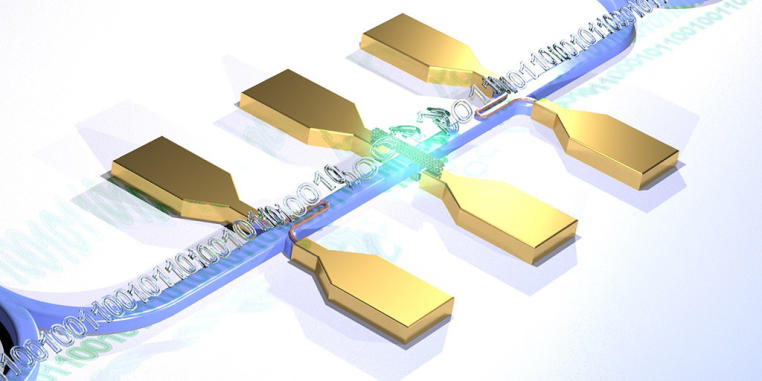
Optical quantum computers are what people are pinning their hopes on for tomorrow’s computer technology – whether for tap-proof data encryption, ultrafast calculations involving enormous quantities of data or so-called quantum simulation, which allows highly complex systems to be reproduced on the computer. So far, experiments researching into the applicability of this technology have filled entire laboratory rooms. In order to use this technology in a meaningful way, however, it needs to be accommodated in a very small space. For the first time researchers have now succeeded in putting a complete quantum optical set-up on a chip. This meets one requirement for making it possible to use photonic circuits for quantum computers.
The results have been published in the current issue of the “Nature Photonics” journal. The light source which the researchers used for the quantum photonic circuit was a special nanotube made of carbon. These have a diameter which is a hundred thousand times smaller than a human hair and they emit single light particles (photons) when stimulated by means of laser light. These photons are also known as light quanta, which explains the term “quantum photonic”. The fact that the carbon tubes emit single photons makes them attractive as an ultra-compact light source for optical quantum computers. The scalability of a system – in other words, the possibility of miniaturizing components in order to increase the quantity – is, however, the precondition for using the technology for high-performance computers, all the way up to optical quantum computers.

In an effort to find a way to carry out computations in ways similar to the human brain, researchers from the group of Prof. W. van der Wiel have found a loosely organized clump of gold nanoparticles can be made to do calculations. The study was published in a recent issue of Nature Nanotechnology. The team laid out a few tens of gold nanoparticles, each about 20 nanometers across, in a rough circle and surrounded them with eight electrodes. Using a genetic algorithm, they identified the combinations of voltages that enabled computations to happen. The voltages cause the gold nanoparticles to form a network of transistors that operate in parallel, similar to the way neurons work in the brain. The researchers say this approach is less energy-intensive than traditional silicon computer chips. Although the particles had to be cooled to just above absolute zero in their experiments, researcher Wilfred van der Wiel notes the need to cool the particles decreases with their size, so it could be possible to scale them down to the point they could operate at room temperature. Van der Wiel says the research could be used to create specialized processors that will be able to tackle problems such as pattern recognition that conventional chips struggle with.