 |


![]()
Name:
Molnár István Gábor
Diploma / M.Sc degree:
Budapest University of Technology and Economics, Hungary
(June 2012)
PhD Project:
Emulating enzymes with small molecule catalyst
Abstract of Research Project
The Gilmour group's predominant research interest has been driven by molecular pre-organization and the strategic use of the fluorine atom for this purpose. Fluorinated organic compounds possess unique features which arise from the high electronegativity (Χ=4) of the fluorine atom and its highly polarized Cσ+-Fσ- bond. Among these features probably the most well described is the so called gauche effect, which examines the preference of the gauche conformation versus the anti conformation. This effect is based on the phenomena that between a low-lying anti-bonding orbital (e.g. σ*C-F) and an anti-periplanar positioned, electron donating bonding orbital (e.g. σC-H) a stabilising hyperconjugative interaction can occur (see Figure 1). In the case of a β-fluoroiminium ion that gauche conformational preference is more significant, because of the occurring electrostatic/charge dipole interactions between the partially negative fluorine and the positively charged nitrogen atom (see Figure 1). In the case of a complex, non-symmetric, fluorine containing compound the occurring other stabilizing (e.g. π-stacking) or destabilizing (e.g. steric repulsion) effects make the discrimination between the two possible gauche conformations possible.
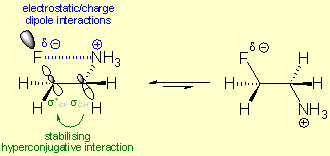
Figure 1: The Fluorine Iminium-Ion Gauche effect
The gauche effect found in fluorinated organic compounds can be easily utilized as an effective tool in the field of molecular pre-organization which recognized by Gilmour et al. The basic idea which has been inspired by nature’s most prominent catalysts - enzymes, was to create a conformationally dynamic structure, which through a substrate-binding event would rigidify into a well-defined conformation. As the target reaction the secondary amine-catalyzed transformation of α,β-unsaturated aldehydes was chosen. Through the reversible formation of the β-fluoroiminium ion intermediate the substituent on the fluorine-bearing carbon atom (e.g. Ph) can be positioned in space in such a way that one of the two possible reaction pathways of the nucleophile is sufficiently blocked and allowing only for the formation of the desired enantiomer. The first catalyst which was designed based on these previous considerations and fully characterised by the group was the (S)-2-(fluoro diphenylmethyl)-pyrrolidine.
The aim of my doctoral research is to design novel, conformationally labile, fluorinated, small molecule catalysts which upon a substrate binding event rigidify; these catalysts should then be tested in a variety of different reactions.
Publications
I. G. Molnár, E.-M. Tanzer, C. Daniliuc, R. Gilmour
Enantioselective Aziridination of Cyclic Enals Facilitated by the Fluorine-Iminium Ion Gauche Effect
Chem. Eur. J. 19 (2013), 1-8.