|
|
|
Free Neuropathology 5:12 (2024) |
|
Letter |
|
Brainstem inflammation in sudden unexpected death in infancy and childhood (SIDS/SUDC) |
|
|
Corresponding author: |
|
Submitted: 2 April 2024 |
|
Keywords: SIDS, SUDC, Brain inflammation, Brainstem, Neuroinflammation, Microglia |
|
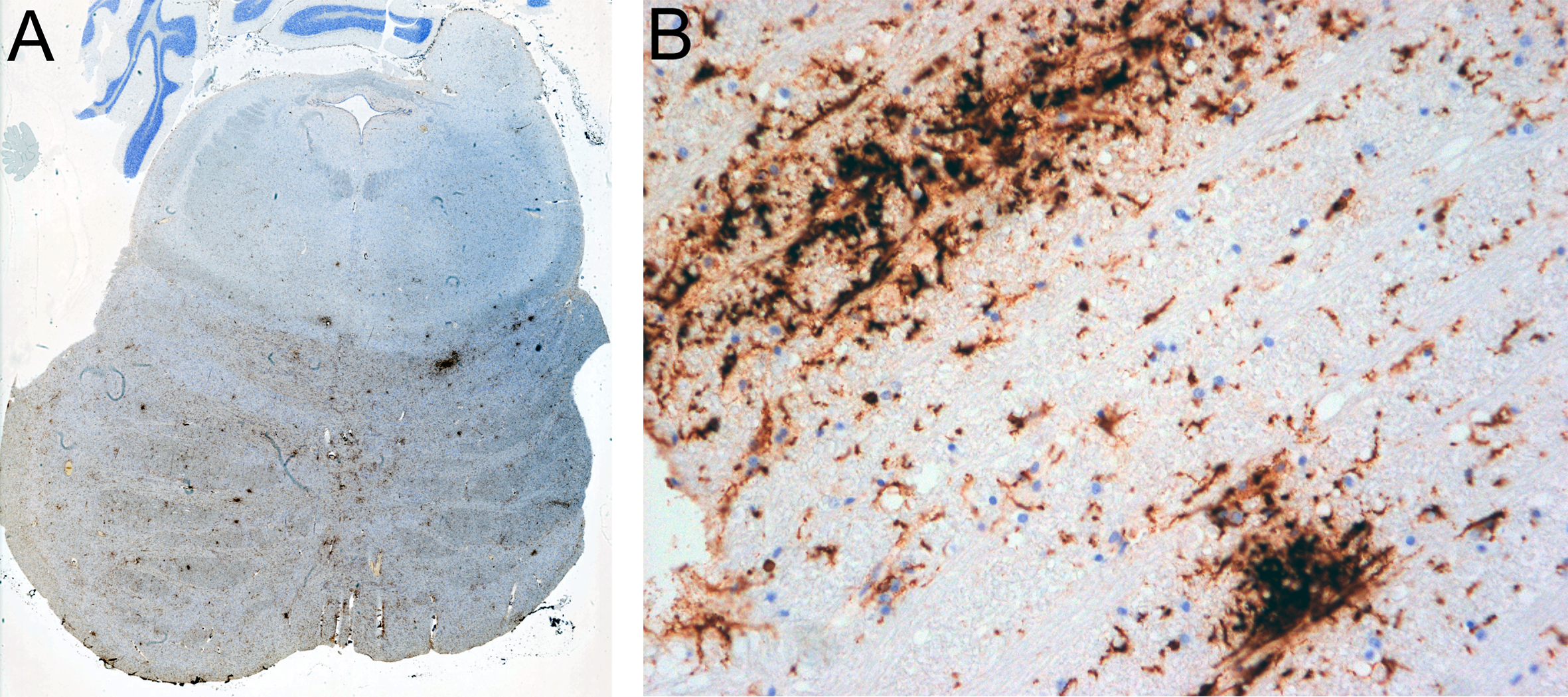
Sudden Unexpected Infant Death (SUID) is the leading cause of death in the first year of age in developed countries [4] and a mystery in medicine still to be clarified. Its major component is Sudden Infant Death Syndrome (SIDS) [4], defined as “sudden death of an infant under one year of age that remains unexplained after a thorough case investigation, including performance of a complete autopsy, examination of the death scene and review of the clinical history” [3]. Identification of risk factors in the infant sleep environment resulted in SIDS being perceived as a sleep accident, with some decrease in prevalence by providing appropriate warnings [4]. However, renewed scientific efforts to identify biologic causes were recently urged [4]. Most research suggests heterogeneity in the etiology of SIDS and its analogue after the first year of life, Sudden Unexpected Death in Childhood (SUDC). There is evidence that slight infection and an activated immune system are involved, summarized in [9]. Most recently a “multiomics” study renewed interest in brain inflammation being responsible for some SIDS cases, demonstrating increased neopterin, a biomarker for activated macrophages considered to indicate neuroinflammation, in CSF of 6 among 71 SIDS deaths; one case was studied in more detail, but histology was suboptimal for interpretation [10]. So, what is the pathological substrate in such cases? Prompted by that paper [10], one of us (HB) reviewed his personal forensic neuropathological archive for brains with SIDS or SUDC, excluding trauma, epilepsy or pre‑existing pathology. Since 2010, when immunocytochemistry for microglial activation markers has been regularly employed in the forensic setting, 6 cases with a clinical diagnosis or suspicion of SIDS (n = 3, ages 3.5, 5 and 7 months) or SUDC (n = 3, ages 15, 35 and 36 months) were revisited. These children were found dead in bed 40 minutes, or 3, 3, 5, 8 hours after last seen alive, respectively; became suddenly blue and had cardiac arrest. General autopsy did not reveal any definite cause of death. Neuropathologically, two cases showed multiple microglial nodules in the brainstem, two cases more diffuse and widespread microglial activation of basal brain regions including the brainstem, one a mixture of these, and another none of these. Intensity ranged from few small nodules to abundant (Fig. 1A), including confluent nodules (Fig. 1B), whereas lymphocytic infiltration was absent. We believe that this type of neuropathology perfectly fits as substrate of the reported neopterin increase in CSF [10]. It indicates early pathology as to be expected in sudden death, and is observational but plausible as a cause for sudden cardio-respiratory dysfunction due to affection of vital brainstem centers, possibly by local excess of proinflammatory mediators produced by activated microglia, as a neuro‑cytokine connection [9].
Figure 1. Brain sections of a 35-month-old, found dead 3 hours after last seen alive. A. Many labeled microglial nodules (tiny brown/dark patches) in a transverse section of the pons. B. Single and larger confluent nodules composed by large-bodied activated microglia in the tegmentum of the medulla oblongata. A and B immunohistochemistry with anti-HLA-DR (MHC II, clone CR3/43 DAKO, diluted 1:400), performed in a DAKO Autostainer with DAKO Envision Kit and diaminobenzidine (DAB) as chromogen, with hematoxylin as counterstain. A x 2, B x 200. The selective mode of our case recruitment (see Declarations below) precludes any sound conclusion on the frequency of these changes. Regardless, the phenomenon does occur, is frequent in our series, and substantiates the heterogeneity of SIDS/SUDC. However, neuroinflammation is not specifically listed among the - mainly unspecific - neuropathological abnormalities described in SIDS [3]. Possible reasons for underrating might be that, first, such a finding does no longer formally comply with the definition of SIDS that requires absence of pathology. Second, and in our view more importantly, routine autopsy work-up may not have focused on visualization of activated microglia that requires appropriate markers, since routine stains such as H&E, luxol fast blue or immunohistochemistry for GFAP may fail to reveal such pathology. For such visualization, Iba1 has been considered as a general marker of microglia by Ambrose et al. [1], but their study did not equate hypertrophic Iba1+ microglia with activation. Moreover, in addition to HLA-DR (MHC-II), CD68 has been used for visualization of activated microglia [1, 10]. However, it is not our experience that CD68 is equally suited as HLA‑DR to show early microglial activation; instead, in our hands CD68 is particularly valuable when microglia convert to macrophages, i.e., as a marker of phagocytosis. Clearly there is more work to be done in this area. Regardless of how valuable the visualization of microglia is, it is necessary to emphasize that their activation just indicates a quick, highly sensitive, but primarily unspecific reaction to pathogenic stimuli, ranging from hypoxia and vascular impairment to trauma, infections and inflammation, neurodegeneration and even tumors. In a detailed study of infants, microglial heterogeneity in regional distribution and activation across the brain was described as characteristic, including a series of SIDS/SUDC cases with immunohistochemistry for Iba1, CD68 and HLA-DR; however, the brainstem had the “smallest” activation, and HLA-DR was most infrequently observed across brain regions; in the context of SIDS, the dentate gyrus was found as region of interest for microglial activation [1], rather than the brainstem. In earlier publications, microglia in infant brains including SIDS and SUDC have been studied in various regions by various methods with variable results (Supplementary Table 1 in [1]). The nucleus tractus solitarii in SIDS was found to harbor a significant increase of reactive astrocytes, a significantly higher percentage of necrotic cells, and an increase of microglial cells [2]. In contrast to these previous publications, our small series demonstrated multiple classical microglial nodules and/or more diffuse microglial activation, with focus on the brainstem but not other brain regions. In absence of other pathogenic clues, we believe that such neuropathology suggests an early inflammatory process that has not yet progressed to elicit lymphocytic infiltration and astrogliosis. It would be well in accordance with a viral infection as early brainstem encephalitis, although the virus type is not evident. Indeed, 4 of our 6 cases were reported to have had a mild cold, what has been considered as a risk factor for SIDS; a range of viruses has been reported in SIDS [9]. In our case illustrated in Fig. 1, a tracheal swab was positive for parainfluenza 2 virus, and human parechovirus 3 was found by others in one case [10]. Previously, meningeal inflammation in SIDS/SUDS was described as lymphocytic infiltration, interpreted as unspecific [5], and lymphocytic pleocytosis in postmortem CSF [7]. Massive pathology of brain edema and lymphocytic meningitis was present in 2 SUDC cases positive for influenza A or adenovirus [6]. SARS-CoV-2 infection in infants (although possibly coincidental) may present as SUID with subtle and unspecific neuropathology, and one case was described to have prominent groups of microglia and mononuclear cells in the brainstem [11]. In any case, a thorough investigation of SIDS/SUDC should include a complete brain autopsy with immunocytochemistry for activated microglia. Research for additional signs of neuroinflammation and deep sequencing for pathogen discovery as recommended [8] will hopefully improve our understanding of the nature of these tragedies. DeclarationsCompeting interestsHB is a member of the Editorial Board of Free Neuropathology. All three authors are providing (neuro)pathological examinations in forensic cases as sworn‑in experts, ordered by District Attorneys or Courts in Austria. Brains are examined on suggestion by Departments of Forensic Medicine performing complete legal autopsies. HB has no influence on the decision to submit such brains or not, and there are other neuropathological experts available as well. Financial disclosureOur expert forensic assessments are paid for by the contracting authority, according to legally fixed rates that are divided between the examiner and the university. We have no other financial disclosure to make. No funds, grants, or other support was received. Ethics approvalThis is a retrospective analysis of anonymized patient data obtained in routine care. The Paris Lodron University of Salzburg Research Ethics Committee raised no objections to the publication of the results. AcknowledgementsWe are grateful to Dr. Sigrid Klotz and Prof. Romana Höftberger for help with photomicrographs, to Mrs. Carmen Haider and Susanne Schmid for archival help, and to the staff of the Histology Lab for excellent technical work-up, all at the Division of Neuropathology and Neurochemistry (Obersteiner Institute), Department of Neurology, Medical University of Vienna, Austria. References 1. Ambrose N, Rodriguez M, Waters KA, Machaalani R (2020) Microglia in the human infant brain and factors that affect expression. Brain Behav Immun – Health 7: 100117. https://doi.org/10.1016/j.bbih.2020.100117 2. Biondo B, Magagnin S, Bruni B, Cazzullo A, Tosi D, Matturri L (2004) Glial and neuronal alterations in the nucleus tractus solitarii of sudden infant death syndrome victims. Acta Neuropathol 108/4: 309-318. https://doi.org/10.1007/s00401-004-0895-2 3. Folkerth RD, Del Bigio MR (2015) Disorders of the Perinatal Period. In: Love S, Budka H, Ironside JW, Perry A (eds) Greenfield’s Neuropathology, 9th ed, vol 1, pp. 210-269. CRC Press, Boca Raton London New York. https://doi.org/10.1201/9781315382715 4. Goldstein RD, Kinney HC, Guttmacher AE (2022) Only halfway there with sudden infant death syndrome. N Engl J Med 386/20: 1873-1875. https://doi.org/10.1056/nejmp21192212 5. Jack E, Haas E, Haddix TL (2019) Evaluation of the presence and distribution of leptomeningeal inflammation in SIDS/SUDI cases and comparison with a hospital-based cohort. Childs Nerv Syst 35/12: 2391-2397. https://doi.org/10.1007/s00381-019-04268-z 6. Krous HF, Chadwick AE, Miller DC, Crandall L, Kinney HC (2007) Sudden death in toddlers with viral meningitis, massive cerebral edema, and neurogenic pulmonary edema and hemorrhage: report of two cases. Pediat Develop Pathol 10/6: 463-469. https://doi.org/10.2350/06-08-0156.1 7. Morris JA, Harrison LM, Telford DR (2012) Postmortem cerebrospinal fluid pleocytosis: a marker of inflammation or postmortem artifact? Internat J Pediat: 964074. https://doi.org/10.1155/2012/964074 8. Nath A (2024) Editorial: Brainstem Encephalitis as a Cause of Sudden Infant Death Syndrome. JAMA Neurol 81/3: 231-232. https://doi.org/10.1001/jamaneurol.2023.5384 9. Opdal SH (2018) Cytokines, Infection, and Immunity. Chapter 30 in: Duncan JR, Byard RW (eds.) SIDS. Sudden Infant and Early Childhood Death: The Past, the Present and the Future, pp. 689-710. Adelaide (AU): University of Adelaide Press. https://doi.org/10.20851/sids 10. Ramachandran PS, Okaty BW, Riehs M, Wapniarski A, Hershey D, Harb H, Zia M, Haas EA, Alexandrescu A, Sleeper LA, Varga SO, Gorman MP, Campman S, Mena OJ, Levert K, Hyyland K, Goldstein RD, Wilson MR, Haynes RL (2024) Multiomic analysis of neuroinflammation and occult infection in sudden infant death syndrome. JAMA Neurol 81/3: 240-247. https://doi.org/10.1001/jamaneurol.2023.5387 11. Stram MN, Seifert AC, Cortes E, Akyatan A, Woodoff-Leith E, Borukhov V, Tetlow A, Alyemni D, Greenberg M, Gupta A, Krausert A, Mecca L, Rodriguez S, Stahl-Herz J, Guzman MA, Delman B, Crary JF, Dams-O’Connor K, Folkerth RD (2022) Neuropathology of pediatric SARS-CoV-2 infection in the forensic setting: novel application of ex vivo imaging in analysis of brain microvasculature. Front Neurol 13: 894565. https://doi.org/10.3389/fneur.2022.894565 |