„Role of complex-modified N-glycans on secretory glycoproteins in higher plants“
| Arabidopsis thaliana „complex glycan minus“ (cgl) mutant | 3D picture of a leaf mesophyll cell in an intact transgenic tobacco plant (magnification ca. 640-times). |
 |
Hinweis: Sie benötigen den Adobe Flash Player und müssen JavaScript in Ihrem Browser aktivieren, um dieses Video im Browser betrachten zu können. Mit einem Klick auf das Bild, können Sie das Video herunterladen. |
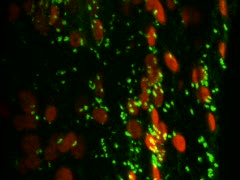
| The picture shows a C5#5 N („non-stainer“) plant during a back-cross. Coloured threads mark already treated flowers (i.e. after removing immature anthers, the stigmas are pollinated with Wildtype). | Due to autofluorescence, chloroplasts appear red. The Golgi is marked in green, i.e. through constitutive expression of an amino-terminal fusion of the GnTI-membrane anchor and the "green fluorescent protein" (GFP). The picture was created by Dr. Liebe (LEICA) with the confocal Laser-Scanning Microscope (cLSM) Leica TCS SP2 / AOBS. |
In the lumen of the Endoplasmic Reticulum (ER), secreted glycoproteins are not only folded but also become “core” glycosylated (with „high mannose“ N-glycans). Like in all other Eukaryotes, this is a pre-requisite for correct folding of glycoproteins prior to subsequent vesicle transport to the Golgi Apparatus (GA). Upon arrival in the GA, ER-type „high mannose“ glycans that are accessible at the protein surface become modified to „complex type“ N-glycans, carrying plant-specific β1,2-xylose and “core” α1,3-fucose residues (that are immunogenic for certain mammals and humans). In Arabidopsis cgl1 („complex glycosylation-less“) mutants with defective GnTI (N-acetyl-Glucosaminyl-Transferase I) in the cis-Golgi, however, this early block did not result in obstruction of general plant development (von Schaewen et al., 1993). The GnTI defect was phenocopied in agronomically important potato and tobacco plants by means of “antisense” or RNAi approaches (Wenderoth & von Schaewen, 2000; Kaulfürst-Soboll et al., 2011a), and found to be largely tolerated. During the past years and in cooperation Prof. Hisashi Koiwa (Texas A&M University, USA), we used a collection of Arabidopsis KO mutants to show that (besides enzymatic steps in the ER) also Golgi-resident N-glycan modifications of secreted glycoproteins play important roles for abiotic stress tolerance (Kang et al., 2008; Kaulfürst-Soboll et al., 2011b) and also biotic stress defense (Häweker et al., 2011). We found that certain mutant combinations that are affected in both ER- and Golgi-resident glycosylation defects showed a wide range of defects (already without stress), i.e. from subtle root phenotypes (Frank et al., 2008) to severe developmental obstruction (Kang et al., 2008; von Schaewen et al., 2008). Currently we study, whether this is also the case upon suppression of similar activities in the Solanaceae, and whether/to what extent in planta use (or prevented use) of N-glycosylation sites may affect protein function, stability, and/or targeting of selected glycoprotein candidates.
3rd party Funding:
Deutsche Forschungsgemeinschaft (DFG)
Involved scientists:
Prof. Dr. Antje von Schaewen, Dr. Heidi-Kaulfürst-Soboll, PhD student: Stephan Rips, Master student: Hanna-Elisa Krawczyk (Manuel Frank, Dr. Julia Frank, TA Tanja Herbrig, TA Olessja Becker).
Cooperations:
Hisashi Koiwa, Associate Prof. Texas A&M University, College Station TX, USA. Prof. Dr. Kazuhito Fujiyama, Osaka University, Japan.
