
 |
 |
 |
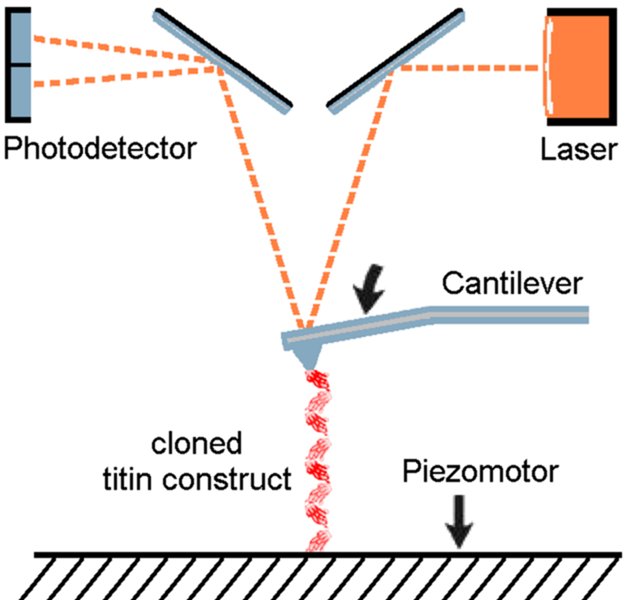
Principle of AFM force spectroscopy |
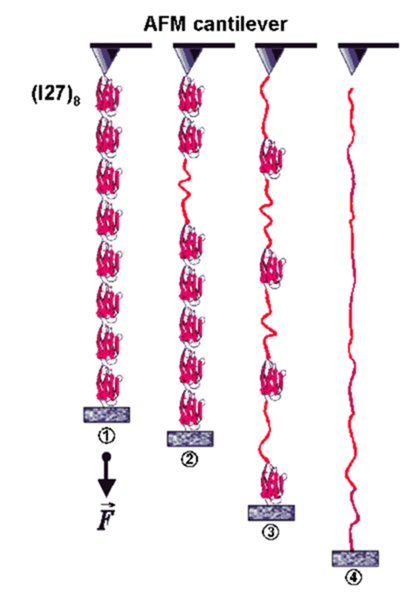
Stretching recombinant titin |
Force-extension sawtooth curve of titin construct |
We use atomic force microscopy (force spectroscopy) to measure the force-extension curves of single, engineered, titin constructs (Li et al., 2002; Linke et al., 2002; Bullard et al., 2004). We have collaborated on this topic with Prof. J.M. Fernandez (Columbia University, New York) (Fernandez lab website). The picture and video clip illustrate that the titin immunoglobulin-module I27 can be used as a "gold standard" in the AFM force measurements. Shown on the left is a schematic of how an engineered Ig I27 octamer is attached to the AFM cantilever and a coverslip surface and is stretched by a force, F. Stages 1 to 4 correspond to the numbers above the force-extension curve on the right. This force trace shows a typical sawtooth pattern, in which the peaks near 200 pN indicate Ig-domain unfolding events. The last peak (4) corresponds to rupture of the polymer from the sites of attachment. The red lines are fits made by using algorithms based on polymer elasticity theory (wormlike chain (WLC) model). One unfolding event is calculated to increase the contour length (L) of the polyprotein by exactly 28.1 nm. This kind of AFM approach has been used to determine the molecular basis of elasticity of individual titin segments (Li et al., 2002). More recently we found evidence that the chaperone alpha-B-crystallin, a member of the small heatshock protein family, stabilizes titin-Ig domains in single-molecule AFM force measurements (Bullard et al., 2004).
References:
Bullard B., C. Ferguson, A. Minajeva, M.C. Leake, M. Gautel, D. Labeit, L. Ding, S. Labeit, J. Horwitz, K. Leonard & W.A. Linke (2004) Association of the chaperone alpha-B-crystallin with titin in heart muscle. J. Biol. Chem. 279:7917-7924. free pdf from publisher
Li H., W.A. Linke, A.F. Oberhauser, M. Carrion-Vazquez, J.B. Kerkvliet, H. Lu, P.E. Marszalek & J.M. Fernandez (2002) Reverse engineering of the giant muscle protein titin. Nature. 418:998-1002. free pdf available here plus online supplement, and News coverage in J. Cell Biol.
Linke W.A., M. Kulke, H. Li, S. Fujita-Becker, C. Neagoe, D.J. Manstein, M. Gautel & J.M. Fernandez (2002) PEVK domain of titin: an entropic spring with actin-binding properties. J. Struct. Biol. 137:194-205. free pdf available here
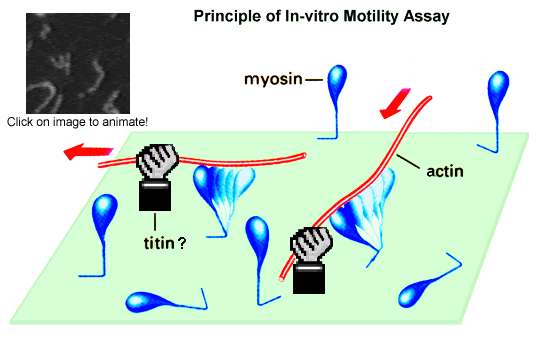
In the actomyosin in-vitro motility assay, fluorescently labeled
actin filaments move
past myosin molecules (or only myosin heads) attached to a
nitrocellulose-coated cover
glass surface. Movement is initiated by addition of magnesium-ATP. The
actin sliding
velocity is an indicator of the efficiency of the molecular motor,
myosin. Actin-binding
protein can alter the sliding speed, as we have recently demonstrated
for cloned titin
constructs--specifically, the PEVK-domain of titin (Kulke et al., 2001;
Linke et al.,
2002). The weak actin-PEVK interaction causes viscous drag during
length changes of
sarcomeres, which affects the passive stiffness of myofibers. The
actin-PEVK interaction
is partially inhibited by physiological calcium concentrations, whereas
Ca2+-S100 protein
or Ca2+-calmodulin did not affect this interaction (Kulke et al., 2001).
References:
Kulke M., S. Fujita-Becker, E. Rostkova, C. Neagoe, D. Labeit, D.J. Manstein, M. Gautel & W.A. Linke (2001) Interaction between PEVK-titin and actin filaments: origin of a viscous force component in cardiac myofibrils. Circ. Res. 89:874-881. pdf available free from publisher, plus online data supplement
Linke W.A., M. Kulke, H. Li, S. Fujita-Becker, C. Neagoe, D.J. Manstein, M. Gautel & J.M. Fernandez (2002) PEVK domain of titin: an entropic spring with actin-binding properties. J. Struct. Biol. 137:194-205. free pdf available here