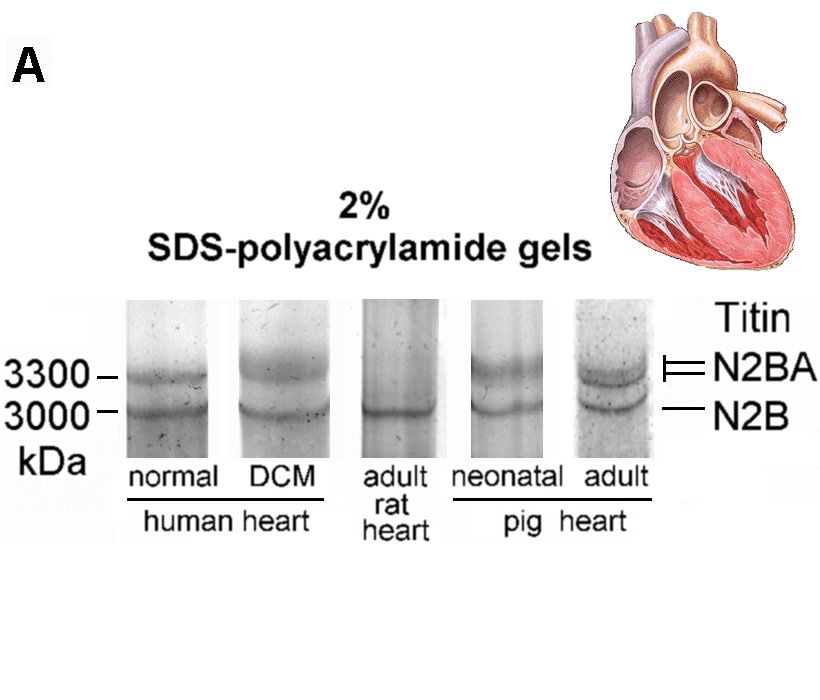
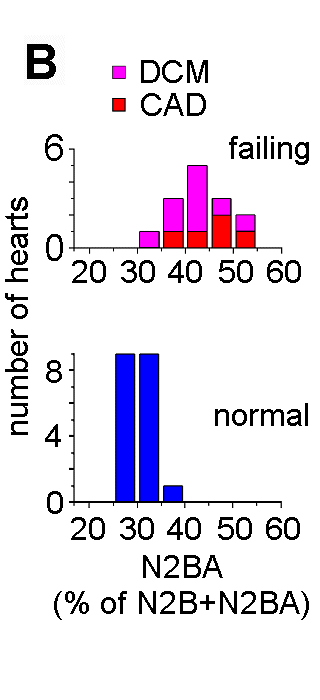
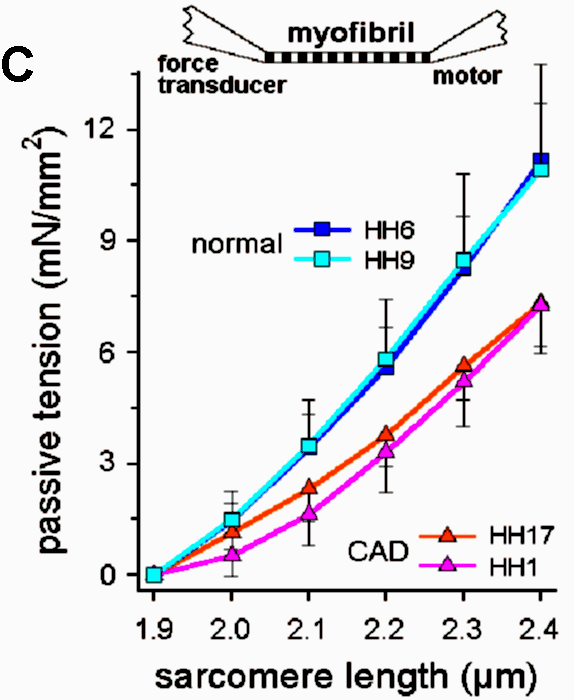
Recent investigations using quantitative RT-PCR and high-resolution SDS-polyacrylamide gel electrophoresis showed that the titin isoform expression pattern is modified dramatically during perinatal heart development (Opitz et al., 2004). To a lesser degree, a titin-isoform shift occurs also in diseased myocardium, including human heart (Neagoe et al., 2002). Titin-stiffness is tuned by changing both the length of titin in the I-band and the expression ratio of longer, more compliant N2BA-isoforms relative to short, stiff N2B-isoform. In the perinatal heart a shift occurs from very long embryonic/fetal N2BA-titin isoforms (3.6-3.7 MDa) towards shorter N2BA-isoforms and a predominant N2B-isoform (3.0 MDa); these changes lead to increased titin-based stiffness in the postnatal heart (Opitz et al., 2004). In contrast, iIn end-stage failing human myocardium, a shift towards higher proportions of long N2BA-isoforms causes decreased myofibrillar passive stiffness (Neagoe et al., 2002; Makarenko et al., 2004).
 |
 |
 |
Makarenko,
I., C.A. Opitz, M.C. Leake, C. Neagoe, M. Kulke, J.K. Gwathmey, F. del
Monte, R.J. Hajjar & W.A. Linke (2004) Passive stiffness changes
caused by upregulation of compliant titin isoforms in human dilated
cardiomyopathy hearts.
Circ. Res. 95:708-716. free pdf
from publisher and free online
data
supplement
Neagoe C., M. Kulke, F. del Monte, J.K. Gwathmey, P.P. de Tombe, R.J. Hajjar & W.A. Linke (2002) Titin isoform switch in ischemic human heart disease. Circulation. 106:1333-1341. free pdf from publisher
Opitz, C.A., M.C. Leake, I. Makarenko, V. Benes & W.A. Linke (2004) Developmentally regulated switching of titin size alters myofibrillar stiffness in the perinatal heart. Circ. Res. 94:967-975. free pdf from publisher , online data supplement , and Editorial
We generated a "knock-in" mouse model replacing native cardiac myosin-binding protein-C with N-terminally truncated cMyBP-C and measured the calcium-sensitivity of active force in skinned cardiac fibers (Witt et al., 2001). An increased calcium sensitivity was found, likely due to higher mobility of myosin heads after truncation of cMyBP-C (see Figure).
Witt, C.C., B. Gerull, M.J. Davies, T. Centner, W.A. Linke & L.
Thierfelder (2001)
Hypercontractile properties of cardiac muscle fibers in a knock-in
mouse model of cardiac
myosin-binding protein-C. J. Biol. Chem. 276:5353-5359. pdf
available free from
publisher.
A novel "slack test" method developed by us has allowed the
quantitation of
the passive recoil speed of titin in rabbit psoas myofibrils (Minajeva
et al., 2002) and
human heart myofibrils (Opitz et al., 2003). In the presence of
titin-based passive force,
titin elastic recoil importantly contributes to the shortening velocity
of muscle fibers.
Minajeva, A., C. Neagoe, M. Kulke & W.A. Linke (2002) Titin-based
contribution to
shortening velocity of rabbit skeletal myofibrils. J. Physiol.
540:177-188. pdf
available free from
publisher.
Opitz, C.A., M. Kulke, M. C. Leake, C. Neagoe, H. Hinssen, R.J. Hajjar & W.A. Linke (2003) Damped elastic recoil of the titin spring in myofibrils of human myocardium. Proc. Natl. Acad. Sci. USA. 100:12688-12693. free pdf available from Pubmed Central plus online supplement.