![]()
|
|
|
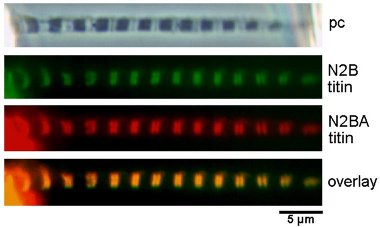
The single myofibril is the minimum preparation that retains the natural architecture of the sarcomere. As such, it has some important advantages over the larger preparations that also retain the lattice. First, interpretation is direct: measured tension is borne fully and completely by the sarcomeres under observation; thus, tension and sarcomere dynamics can be related to one another without assumption. Second, the preparation is practically molecular in scale. Results can be interpreted mechanistically with fewer hidden assumptions than is true with larger preparations.
 |
|
We have completed many studies reporting mechanical properties of
single myofibrils.
Both active and passive forces have been the focus of attention. We
showed that single
cardiac myofibrils generate active tension values of ~0.15 N/mm2,
comparable to larger
cardiac-muscle preparations when the difference in myofibrillar
cross-sectional area is
considered. The level of passive tension was shown to be similar in
single cardiac
myofibrils and trabeculae or papillary muscles over a physiological
sarcomere-length range
from approximately 1.9 to 2.2 µm, indicating the important
contribution of myofibrils to
the passive stiffness of whole myocardium. The single myofibril was
also used to
investigate the mechanical properties of titin filaments in vertebrate
muscle or
titin-like proteins in insect flight muscle; titins were found to be
largely responsible
for the passive myofibrillar stiffness. In related experiments we
applied various models
of polymer elasticity theory to mathematically describe the mechanics
of titin in the
myofibril. We found that the passive tension of single cardiac
myofibrils can be
reconstituted using parameters obtained in single-molecule AFM
mechanics on titin domains.
More recently, we have compared the passive tension in single
myofibrils of normal and
chronically diseased human myocardium; decreased titin-based passive
force was seen in
myofibrils of failing myocardium. In a different set of experiments, we
have used the
single-myofibril preparation to measure the titin-based contribution to
shortening
velocity of skeletal muscle fibers and to quantify the speed of titin
elastic recoil in
single human cardiac myofibrils.
Kulke, M., C. Neagoe, B. Kolmerer, A. Minajeva, H. Hinssen, B. Bullard & W.A. Linke (2001) Kettin, a major source of myofibrillar stiffness in Drosophila indirect flight muscle. J. Cell Biol. 154:1045-1057. News coverage in J. Cell Biol. and pdf available free from publisher.
Linke, W.A., V.I. Popov & G.H. Pollack (1994) Passive and active tension in single cardiac myofibrils. Biophys. J. 67:782-792. free pdf from Pubmed Central
Linke, W.A., M. Ivemeyer, N. Olivieri, B. Kolmerer, J.C. Rüegg & S. Labeit (1996) Towards a molecular understanding of the elasticity of titin. J. Mol. Biol. 261:62-71. free pdf here
Linke, W.A., M. Ivemeyer, P. Mundel, M.R. Stockmeier & B. Kolmerer (1998a) Nature of PEVK-titin elasticity in skeletal muscle. Proc. Natl. Acad. Sci. USA. 95:8052-8057. pdf available free from publisher.
Linke, W.A., M.R. Stockmeier, M. Ivemeyer, H. Hosser & P. Mundel (1998b) Characterizing titin's I-band Ig domain region as an entropic spring. J. Cell Sci. 111:1567-1574. pdf available free from publisher.
Linke, W.A., D.E. Rudy, T. Centner, M. Gautel, C. Witt, S. Labeit & C.C. Gregorio (1999) I-band titin in cardiac muscle is a three-element molecular spring and is critical for maintaining thin filament structure. J. Cell Biol. 146:631-644. pdf available free from publisher.
Minajeva, A., C. Neagoe, M. Kulke & W.A. Linke (2002) Titin-based contribution to shortening velocity of rabbit skeletal myofibrils. J. Physiol. 540:177-188. pdf available free from publisher.
Neagoe C., M. Kulke, F. del Monte, J.K. Gwathmey, P.P. de Tombe, R.J. Hajjar & W.A. Linke (2002) Titin isoform switch in ischemic human heart disease. Circulation. 106:1333-1341. free pdf from publisher
Opitz, C.A., M. Kulke, M. C. Leake, C. Neagoe, H. Hinssen, R.J. Hajjar & W.A. Linke (2003) Damped elastic recoil of the titin spring in myofibrils of human myocardium. Proc. Natl. Acad. Sci. USA. 100:12688-12693. free pdf from pubmed central plus online supplement
Li H., W.A. Linke, A.F. Oberhauser, M. Carrion-Vazquez, J.G. Kerkvliet, H. Lu, P.E. Marszalek & J.M. Fernandez (2002) Reverse engineering of the giant muscle protein titin. Nature. 418:998-1002. News coverage in J. Cell Biol. and free pdf available here, plus online supplement.
Linke, W.A. & J.M. Fernandez (2002) Cardiac titin: Molecular basis of elasticity and cellular contribution to elastic and viscous stiffness components in myocardium. J. Muscle Res. Cell Motil. 23:483-497. free pdf available here.
Linke, W.A., M. Ivemeyer, P. Mundel, M.R. Stockmeier & B. Kolmerer (1998) Nature of PEVK-titin elasticity in skeletal muscle. Proc. Natl. Acad. Sci. USA. 95:8052-8057. pdf available free from publisher.
Minajeva, A., M. Kulke, J.M. Fernandez & W.A. Linke (2001) Unfolding of titin domains explains the viscoelastic behavior of skeletal myofibrils. Biophys. J. 80:1442-1451. pdf available free from publisher.