Advanced Electrolyte Additives Improve Performance and Lifetime of Silicon based Anodes
A team of MEET Battery Research Center, the Institute of Organic Chemistry and the International Graduate School BACCARA has developed a new concept for silicon based anodes in lithium-ion battery (LIB) based on a class of electrolyte additives that guarantees high energy density while counteracting degradation. The research team addresses two of the biggest challenges that are currently still preventing the technology's breakthrough.
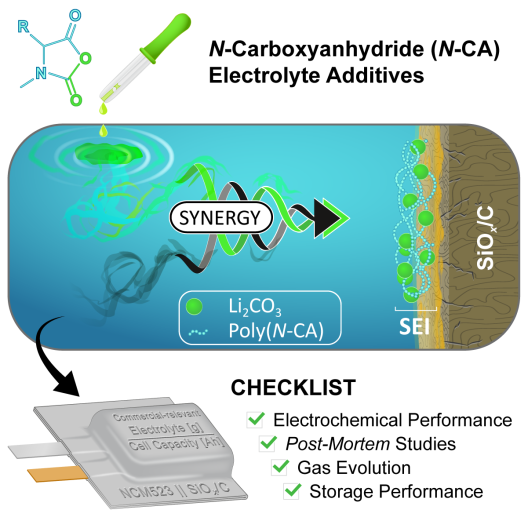
Innovative electrolyte additives protect the electrode
The silicon anode is one of the most auspicious approaches in research on new material concepts for the lithium-ion battery. The lithium-alloying material promises a significant increase in energy density compared to the currently used graphite, as it accumulates up to 10 times more lithium ions. The breakthrough of the silicon anode is currently prevented by a significant expansion of the silicon (up to 300% per volume) during the charging and discharging of the battery. The result is degradation of the electrode combined with a short battery life.
“The increased use of silicon as an anode material is intensively analysed in numerous research projects, but also by industry – especially because the demands on batteries are constantly growing”, emphasises Dr Tobias Placke, Division Manager Materials at MEET Battery Research Center of the University of Münster. In the currently commercially available LIBs with silicon-based anodes, the proportion of the alloying material is less than ten percent, since the batteries would not fulfil the lifetime requirements otherwise.
In order to increase the amount of silicon in the anode, the scientific team has developed a new class of electrolyte additives. Based on N-carboxyanhydrides, the tailor-made design ensures the formation of an effective solid electrolyte interphase (SEI). This interphase between the electrode and the electrolyte counteracts the degradation of the electrode, which significantly increases the number of possible charging and discharging cycles of the battery.
Analytical methods enable deep insights into the reactions in the battery
Using NCM523 II SiOx/graphite lithium-ion cells as an example, the researchers simultaneously found increased energy of the cell due to the silicon-based anode and improved cell lifetime due to the novel additives. Linda Quach, Ph.D. student at the International Graduate School for Battery Chemistry, Characterization, Analysis, Recycling and Application (BACCARA), states: “We developed efficient electrolyte additives, evaluated them carefully on cell level and look into the degradation of the electrode, the formation of the SEI and the gas formation by use of complementary post-mortem analytics.”
The scientists Jan-Patrick Schmiegel, Dr Roman Nölle, Dr Jonas Henschel, Dr Sascha Nowak, Prof. Dr Martin Winter and Dr Tobias Placke from MEET Battery Research Center as well as Prof. Dr Frank Glorius and Linda Quach from the International Graduate School BACCARA and the Institute of Organic Chemistry at the University of Münster, have published the detailed results of their study as an open access article in the journal “Cell Reports Physical Science”.
